The FDA approved secukinumab (Cosentyx) this past week for the treatment of adults with active ankylosing spondylitis and active psoriatic arthritis. These two new approvals are based on the safety and efficacy outcomes from four placebo-controlled Phase 3 trials in more than 1,500 adults with AS or PsA…
Search results for: secukinumab

Phase 3 Trials: Secukinumab for Psoriatic Arthritis & DA-DKP for OA
In a global Phase 3 trial, subcutaneous secukinumab proved safe and effective in patients with psoriatic arthritis. Also, a version of aspartyl-alanyl diketopiperazine, a biologic for knee OA, has entered Phase 3 trials…

Secukinumab Effective for Psoriatic Arthritis
A Phase 3 study found secukinumab may be an effective alternative to anti-TNF therapies for treating psoriatic arthritis, suggesting interleukin 17A may play a role in the disease…
Secukinumab Effective for Treating Psoriatic Arthritis
NEW YORK (Reuters Health)—The anti-interleukin-17A monoclonal antibody secukinumab improves signs and symptoms in patients with psoriatic arthritis, according to results from Novartis’ FUTURE 2 trial. In earlier studies, secukinumab has demonstrated superior effectiveness to placebo and etanercept in improving the signs and symptoms of psoriasis. Dr. Iain B. McInnes from the University of Glasgow in…
Hydrocodone Bitartrate, Secukinumab, Varenicline Updates, Trials, Approvals
Plus, rheumatology drug news, safety updates

Emerging Treatments in Psoriatic Arthritis
At a scientific session of ACR Convergence 2025, Alexis Ogdie, MD, discussed new and forthcoming treatments for psoriatic arthritis.
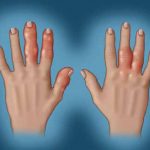
Finding the Panacea: The Management of Psoriatic Arthritis
In a session at EULAR 2025, Dr. Laura Coates discussed the management of PsA, providing insights into the current research and when clinicians may want to consider prescribing specific medications.
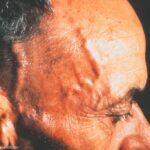
New Study: Upadacitinib Is Treatment Option for Giant Cell Arteritis
Results of the international SELECT-GCA study suggest that upadacitinib may be an effective new oral treatment for giant cell arteritis.
Trends in the Use of DMARDs for Patients with JIA
Yalamanchili et al. describe how trends in disease-modifying anti-rheumatic drug (DMARD) use have evolved for insured, U.S. patients with juvenile idiopathic arthritis. Overall, the study found that from 2000 to 2022 in this patient population the use of biologic and targeted synthetic DMARDs rose, while the use of conventional synthetic DMARDs declined.

The Management of Psoriatic Arthritis: A Review
WASHINGTON, D.C.—As of November 2024, there are 16 biologic disease-modifying anti-rheumatic drugs (bDMARDs) that are FDA approved for the treatment of psoriatic arthritis (PsA). Incredible news, right? But as my fellowship program director used to say, “There’s no free lunch.” This buffet of options is excellent for our patients, but poses challenges to the practicing…
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 11
- Next Page »