St.Clair et al. found that patients with Sjögren’s disease treated with dazodalibep experienced greater improvement in key symptoms of dryness, fatigue & pain than patients who received placebo.


St.Clair et al. found that patients with Sjögren’s disease treated with dazodalibep experienced greater improvement in key symptoms of dryness, fatigue & pain than patients who received placebo.

A study examined the association between TNF inhibitors and neurological demyelinating adverse events in patients with rheumatoid arthritis and spondyloarthritis using cohort data from five Nordic countries. Researchers showed that patients with SpA were more likely to experience adverse events than patients with RA.
Reuters Staff |
NEW YORK (Reuters Health)—Patients with preexisting autoimmune disease (AIDs) are not at increased risk for immune-related adverse events from immune checkpoint inhibitor (ICI) therapy, although these adverse events may be more likely in patients with inflammatory bowel disease (IBD), new research indicates. “Therefore, we encourage physicians not to withhold ICI in most common AIDs. However,…
Will Boggs, MD |
NEW YORK (Reuters Health)—Low-dose methotrexate can be associated with gastrointestinal, pulmonary, infectious, hematologic and other adverse effects, according to an analysis of the Cardiovascular Inflammation Reduction Trial (CIRT). “Methotrexate is not a benign drug, even at dosages used for rheumatic diseases,” Daniel H. Solomon, MD, MPH of Brigham and Women’s Hospital, Boston, tells Reuters Health…
Arthritis Care & Research |
A new case series outlines the treatment and duration of symptoms of 10 patients experiencing the musculoskeletal manifestations of immune-related adverse events. Researchers found these symptoms may last for more than a year, but can generally be treated with low to moderate doses of corticosteroids…

As questions about biosimilar medications swirl among U.S. rheumatologists, a recently released report sheds some light on the European experience with biosimilars—and may offer some important insights for the U.S. market. The report, Biosimilars in the EU: Information Guide for Healthcare Professionals, was released in late April by the European Medicines Agency (EMA) and the…
Systemic sclerosis (SSc) is a rare disease affecting about 49,000 U.S. adults, and it is strongly associated with high levels of morbidity and mortality.1 Of the few available antifibrotic therapies, none is targeted for SSc. However, reason for optimism exists for antifibrotic treatments in early development and clinical trials, says Jörg H.W. Distler, MD, Heisenberg Professor…

Dana Direnzo, MD, Ami A. Shah, MD, MHS, Clifton O. Bingham III, MD, & Laura C. Cappelli, MD, MHS |
A 53-year-old female presented to the clinic for severe polyarticular joint pain and was found to have a seronegative inflammatory arthritis. Six months before, she had completed 10 months of treatment for stage IV metastatic melanoma with the immune checkpoint inhibitors, nivolumab and ipilimumab, achieving complete remission of her cancer. She said that throughout her…
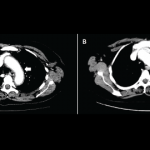
Byung H. Ban, DO, Jayne L. Crowe, MD, & Robert M. Graham, MD |
Ipilimumab (Yervoy) is a monoclonal antibody directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4). It was the first drug to demonstrate a survival benefit in advanced melanoma and was approved by the FDA in 2011.1 By blocking the CTLA-4 receptor, ipilimumab enhances the immune response against tumors via cytotoxic T lymphocyte activation and proliferation.2 However, immunopotentiating…

Some cancer patients taking immune checkpoint inhibitors experience immune-related adverse events. Laura C. Cappelli, MD, MHS, says rheumatologists are natural partners with oncologists to treat this patient population…