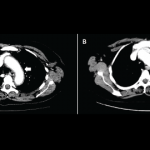
Ipilimumab (Yervoy) is a monoclonal antibody directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4). It was the first drug to demonstrate a survival benefit in advanced melanoma and was approved by the FDA in 2011.1 By blocking the CTLA-4 receptor, ipilimumab enhances the immune response against tumors via cytotoxic T lymphocyte activation and proliferation.2 However, immunopotentiating…