Glucocorticoids remain a prominent part of care for many patients with SLE but can have toxic side effects; this EULAR 2022 session discussed one institution’s approach to lower the dosage.
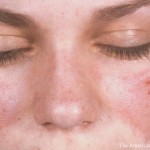

Glucocorticoids remain a prominent part of care for many patients with SLE but can have toxic side effects; this EULAR 2022 session discussed one institution’s approach to lower the dosage.

Clinicians have numerous treatment options for SLE; in a EULAR 2022 session, some of the newer therapies were reviewed.

Elizabeth (Blair) Solow, MD |
ACR CONVERGENCE 2020—Held Nov. 5–9, the ACR’s first fully virtual annual meeting provided participants with a vast repository of new research related to rheumatoid arthritis (RA). To help you sort through the noise, Elizabeth (Blair) Solow, MD, an assistant professor of medicine in the Division of Rheumatic Diseases at UT Southwestern Medical Center, Dallas, offered …
Michael Erman |
NEW YORK, June 4 (Reuters)—Three of the authors of an influential article that found hydroxychloroquine (HCQ) increased the risk of death in COVID-19 patients retracted the study, citing concerns about the quality of the data behind it. The anti-malarial drug has been controversial in part due to support from U.S. President Donald Trump, as well…
Julie Steenhuysen |
CHICAGO (Reuters)—Cancer patients with COVID-19 who were treated with a drug combination promoted by U.S. President Donald Trump to counter the coronavirus were three times more likely to die within 30 days than those who got either drug alone, U.S. researchers reported on May 28. The preliminary results suggest doctors may want to refrain from…
Reuters Staff |
ZURICH (Reuters)—The World Health Organization (WHO) on Tuesday promised a swift review of data on hydroxychloroquine (HCQ), probably by mid-June, after safety concerns prompted the group to suspend the malaria drug’s use in a trial on COVID-19 patients. U.S. President Donald Trump and others have pushed HCQ as a possible coronavirus treatment, but the WHO…
Reuters Staff |
(Reuters)—Malaria drug hydroxychloroquine (HCQ), which U.S. President Donald Trump says he has been taking, is tied to increased risk of death in COVID-19 patients, according to a study published in The Lancet.1 The registry analysis, which included data from 671 hospitals in six continents and over 96,000 patients hospitalized with COVID-19, showed that people treated…
Kylie MacLellan |
LONDON (Reuters)—On May 21, British healthcare workers began taking part in a University of Oxford-led international trial of two anti-malarial drugs to see if they can prevent COVID-19, including one U.S. President Donald Trump says he has been taking. The COPCOV study will involve more than 40,000 frontline healthcare workers from Europe, Africa, Asia and…
Gene Emery |
(Reuters)—The malaria treatment repeatedly championed by U.S. President Donald Trump as a game changer in the fight against the novel coronavirus has again failed to show a benefit in patients hospitalized with COVID-19, according to a recent study. Although the study published in The New England Journal of Medicine had certain limitations, doctors report that…
Michael Erman & Anne Kauranen |
NEW YORK/HELSINKI (Reuters)—Amneal Pharmaceuticals could soon run out of the raw ingredients to make more of the anti-malarial drug hydroxychloroquine (HCQ) that has been touted as a potential treatment for COVID-19 because Finland is keeping the drug for domestic use, according to the generic drugmaker’s chief executives. Amneal has committed to producing about 20 million…