 “Following successful clinical trials and multiple approved therapies for pulmonary arterial hypertension in systemic sclerosis (SSc) over the past two decades, interstitial lung disease (ILD) is currently the most lethal complication of this challenging disease,” says Christopher Denton, MBBS, PhD, FRCP, FMedSci, professor of experimental rheumatology at University College London (UCL) and consultant rheumatologist and head of the Centre for Rheumatology, Royal Free Hospital, London.
“Following successful clinical trials and multiple approved therapies for pulmonary arterial hypertension in systemic sclerosis (SSc) over the past two decades, interstitial lung disease (ILD) is currently the most lethal complication of this challenging disease,” says Christopher Denton, MBBS, PhD, FRCP, FMedSci, professor of experimental rheumatology at University College London (UCL) and consultant rheumatologist and head of the Centre for Rheumatology, Royal Free Hospital, London.
Dr. Denton is the corresponding author of a review that is part of a series on immunology for rheumatologists published in Arthritis & Rheumatology (A&R).1 In this new installment, Dr. Denton and co-authors Nina Goldman, MB Bchir, and Voon Ong, MBBS, PhD, FRCP, review B cells and SSc-ILD.2 Dr. Goldman is a clinical research fellow at UCL and respiratory specialist registrar at NHS Foundation Trust in North East London, and Dr. Ong is a professor of rheumatology and consultant rheumatologist at UCL.
Targeting B Cells in SSc-ILD
“From reading our article, practicing rheumatologists will better understand the reasons behind targeting B cells in SSc-ILD and how it is currently achieved,” Dr. Denton explains. “Our ‘real world’ illustrative case provides readers with an example of clinically meaningful impact.”
The review article centers on a patient case study demonstrating the progression of SSc-ILD, a complication that is a leading cause of mortality and morbidity in SSc. The case study “highlights the diverse range of patients with progressive SSc-ILD where additional biologic therapy can be considered, and also the need to be cognizant of non-ATA [anti-topoisomerase 1 antibody] high risk SSc-ILD antibodies,” the authors write.
Returning to the clinical case throughout the review, the authors discuss the pathogenesis of SSc-ILD, the autoantibody profile of SSc-ILD and the role of B cells in the disease. They detail the treatment options for SSc-ILD, including cyclophosphamide, mycophenolate mofetil, rituximab, nintedanib and tocilizumab, as well as the evidence for B cell therapies and the risks associated with treatment.
Chimeric antigen receptor (CAR) T therapy that “targets CD19 potentially may overcome the limitations of CD20-targeted strategies including rituximab and may target lesional pathogenic antibodies conferring a deep depletive effect,” the authors write.
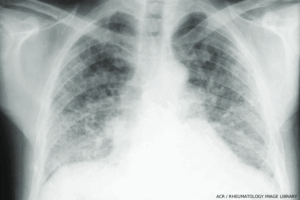
Bilateral interstitial fibrosis involving the lower two-thirds of the lung fields is common in systemic sclerosis. In the lower lung fields are “honeycomb” changes. The heart is not enlarged. (Click to enlarge.)
Along with more than 100 references, the review includes four informative tables. The tables detail the trajectory of the lung function in the patient case study, the main associations of autoantibody profile in SSc-ILD, drugs used for the treatment of the disease and investigational B cell therapies in SSc.
