In both the on-treatment (OT) and intention-to-treat (ITT) analyses, febuxostat was non-inferior to allopurinol for incidence of the primary endpoint (OT analysis: febuxostat 172 patients [1.72 events per 100 patient years]; allopurinol 241 patients [2.05 events per 100 patient years]; hazard ratio 0.85 [95% CI 0.70–1.03], P<0.001; ITT analysis: febuxostat 256 patients [2.05 events per 100 patient years], allopurinol 285 patients [2.29 events per 100 patient years]; hazard ratio 0.89 [95% CI 0.75–1.06], P<0.001).
Two hundred twenty-two (7.2%) patients died in the febuxostat group, compared with 263 deaths (8.6%) in the allopurinol group.
“Because you’re all rheumatologists in here, you all want to know about gout flares,” said Prof. MacDonald. “These weren’t really adjudicated, but patients in both groups had at least one gout flare”: 1,017 patients in the febuxostat group (event rate 17.95 per 100 patient-years) and 1,044 patients in the allopurinol group (event rate 19.85 per 100 patient-years). “But, of course, there’s no placebo group in here, so we don’t know the effectiveness of either of these agents in preventing these flares,” he said.
The study concluded that febuxostat was non-inferior to allopurinol therapy for the primary cardiovascular outcome. “In contrast to previous studies there was no evidence of increased mortality with febuxostat,” he said, “and we think regulators should review febuxostat licensing restrictions.”
In response to a question about the quality of the data from the audience, Prof. MacDonald said, “The concept that we missed deaths or hospitalizations is pretty unlikely. … The records are pretty complete, and we had pretty good follow-up.”
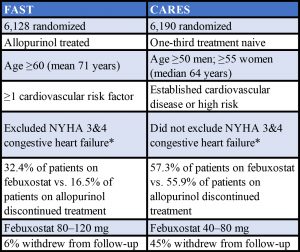 * New York Heart Association Classification. Stage 3: Marked limitation in activity due to symptoms, even during less-than-ordinary activity, (e.g., walking short distances [20–100 m]). Comfortable only at rest. Stage 4: Severe limitations. Experiences symptoms even while at rest. Mostly bedbound patients.
* New York Heart Association Classification. Stage 3: Marked limitation in activity due to symptoms, even during less-than-ordinary activity, (e.g., walking short distances [20–100 m]). Comfortable only at rest. Stage 4: Severe limitations. Experiences symptoms even while at rest. Mostly bedbound patients.
Sources: Adapted from a table presented by Prof. MacDonald and from the Specifications Manual for Joint Commission National Quality Measures (v2018A)
Keri Losavio is the editor of The Rheumatologist.
Study Disclosures: The study was funded by Menarini to fulfil an EMA regulatory commitment; however, Menarini played no part in the running of this study. Menarini received financial support from Ipsen and Teijin Pharma Ltd. for this study. The University of Dundee was the legal sponsor, and received funds for research from Amgen, Astellas, AstraZeneca, GSK, Menarini, Pfizer, Servier, Shire and Takeda. Prof. MacDonald has received speaker or consultancy fees from Novartis, Takeda, Servier, Shire, Astellas, Menarini and AstraZeneca.


