Designua / shutterstock.com
SAN DIEGO—Treatments do exist that can improve the prospects of a patient with the rare disease amyloidosis, but only if clinicians keep the disease in mind and treat the patient before it’s too late, an expert said at the 2017 ACR/ARHP Annual Meeting this past November. He also discussed research that may be close to verifying a new drug treatment for the devastating disease.
“The first and most important thing I want to say to you is, think amyloidosis,” said Sir Mark Pepys, MD, PhD, professor of medicine at University College London in England. Dr. Pepys has been studying amyloidosis for decades, and he founded the UK National Amyloidosis Center, also in London. “Please remember to think amyloidosis when you’re confronted with any clinical case at all. Systemic amyloidosis can mimic just about any clinical presentation you want to talk about, and if you don’t think of it, you’re not going to do the right diagnostic investigations. If you don’t make the diagnosis, the patient will suffer.”
The disease is caused by an accumulation of abnormal protein fibers, called amyloid fibrils, that damage tissues and organs. The body can’t effectively clear these amyloid deposits, which are all but ignored by the normal mechanisms for debris removal. In systemic amyloidosis, the precursors of the fibrils are circulating globular proteins that become misfolded because of an instability that can be inherent, acquired or hereditary.
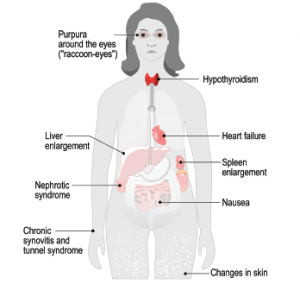
Rheumatologists are most likely to encounter amyloidosis that arises from chronic inflammatory conditions, but it has become less common as these diseases have been managed better. Amyloidosis is not the rarest disease, but “most clinicians have probably never seen a case, or know that they’ve seen a case, in their whole clinical career,” Dr. Pepys said. This means many patients aren’t diagnosed until they have advanced disease, and “as a result, their prognosis is dreadful despite major recent advances in treatment and much better outcomes [available] if you’re diagnosed and treated early in specialty centers.”
Current & Future Treatments
The current idea behind disease treatment is to keep patients alive for as long as possible while efforts are made to remove the precursor protein forming the fibrils and improve the function of failing organs.
Genetic techniques (e.g., antisense oligonucleotides and a drug designed to silence specific messenger RNA) have been developed to target hereditary forms of amyloid deposition and have moved into Phase 3, with some encouraging results, Dr. Pepys said.
Bortezomib, a proteasome inhibitor developed for use in myeloma treatment, has produced responses in many patients with immunoglobulin light chain amyloidosis (AL), so survival from diagnosis can now range from eight to 10 years in many patients. However, Dr. Pepys also said roughly one-quarter of all patients with AL amyloidosis still die within six months of diagnosis. The monoclonal antibody developed for myeloma, daratumumab, also targets the plasma cells that cause AL amyloidosis and, therefore, holds much promise for treating this condition.
But major treatment challenges remain. Although AL patients with low-risk disease can do well if treated aggressively with full-dose chemotherapy, those with intermediate risk might not tolerate full-dose treatment, and their responses can suffer. And those with high-risk disease are even less tolerant of aggressive treatment and have even worse outcomes.
In his research, Dr. Pepys has focused on serum amyloid P component (SAP), a normal blood protein present in everybody, and has used it in both imaging and treatment. SAP is produced in the liver and secreted into the blood, and as it circulates around, it sticks to amyloid if it’s present. When it sticks, there’s much more of it in the amyloid than in the blood—a feature Dr. Pepys called a “dynamic equilibrium.”
By radiolabeling SAP and injecting it into the circulation, clinicians can visualize this equilibrium by imaging amyloid deposits throughout the body. This provides extensive and valuable information not available just from tissue biopsy.
At the UK’s National Amyloidosis Center and elsewhere, researchers began gathering evidence that SAP was not just there by happenstance—it actually contributed to the pathogenesis of amyloidosis, stabilizing the fibrils when it binds to them and even promoting amyloid formation.
After SAP knockout mice were found to take longer to develop amyloid deposition and develop less of it, Dr. Pepys, in collaboration with Roche, created a small molecule to remove SAP from amyloid, on the theory that “if you removed all the SAP from the amyloid, the body would recognize the abnormal fibrils, and macrophages would come along and get rid of them.” Unexpectedly, the drug they produced, miridesap, removed SAP and reduced the amount of SAP in the amyloid deposits. But it did not remove all of it from the deposits, so the amyloid persisted.
Dr. Pepys then realized that after clearing SAP from the blood with miridesap, it would become possible to treat patients with antibodies directed against SAP. The antibodies could home on SAP as a specific marker for amyloid and, once there, trigger the body’s normal, highly effective mechanism for removing abnormal debris from tissue.
The idea worked well in experimental models, and a fully humanized monoclonal anti-SAP antibody, dezamizumab, was produced. Then treatment with miridesap followed by dezamizumab produced unprecedented clearance of amyloid from the tissues and organs of patients in the first human clinical trial. The initial results appeared in the New England Journal of Medicine in 2015, with full results published in Science Translational Medicine in January 2018.1,2
Patients with cardiac amyloidosis were originally excluded from the trial for safety reasons, but subsequently some were treated without any adverse cardiac effects. A Phase 2 trial focused on cardiac amyloidosis is now in progress.
“This really needs to work—if it’s going to be a large-scale drug—in patients with cardiac amyloidosis,” said Dr. Pepys, who is a shareholder in a company that owns the miridesap patents and the patents on his invention of miridesap plus anti-SAP antibody, which are licensed to GlaxoSmithKline. “Because that’s what kills most of the patients with systemic amyloid.”
Thomas R. Collins is a freelance writer living in South Florida.
References
- Richards DB, Cookson LM, Berges AC, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015 Sep 17;373(12):1106–1114.
- Richards DB, Cookson LM, Barton SV, et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018 Jan 3;10(422).