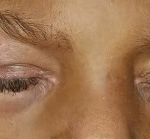
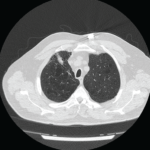
Clinically amyotrophic dermatomyositis (CADM), a subset of dermatomyositis (DM), is a rare autoimmune disease characterized by typical DM cutaneous findings (e.g., heliotrope rash, Gottron papules, Gottron sign) without evidence of myositis.1 The incidence of DM and CADM is approximately 9.63 per 1 million people and 2.08 per 1 million people, respectively.2 The association with development of interstitial lung disease (ILD) and an increased risk of malignancy highlights the importance of prompt diagnosis, disease management and appropriate malignancy screening.
Due to the rarity of CADM, heterogeneity of disease manifestation and recalcitrant nature of the disease, treatment remains challenging; possible overlap with other autoimmune diseases complicates management. However, the advent of immunotherapy has introduced new possibilities in modifying disease progression.3
Here, we describe a case of a patient with CADM, associated ILD and concurrent rheumatoid arthritis (RA) who was successfully treated with a combination of conventional and biologic therapy.
Case Description
Our patient is a 65-year-old woman who works as a fitness instructor. She had no significant past medical history prior to presenting for rheumatological evaluation with a three-month history of rash, and bilateral hand swelling and pain. Her initial symptoms manifested as nonblanching, erythematous, papular lesions along the palmar aspects of the hands and digits, along with periungual erythema. These symptoms resolved after a few weeks, but returned after a month, this time with a new, annular, hyperpigmented patch on the upper left cheek as well. The patient also experienced diffuse swelling, pain and morning stiffness of the proximal and distal interphalangeal joints of the hands. She noted the development of constitutional symptoms, including fatigue and a 30 lb. weight loss, as well as progressive shortness of breath, subjective weakness of the upper extremities and new-onset Raynaud’s phenomenon.
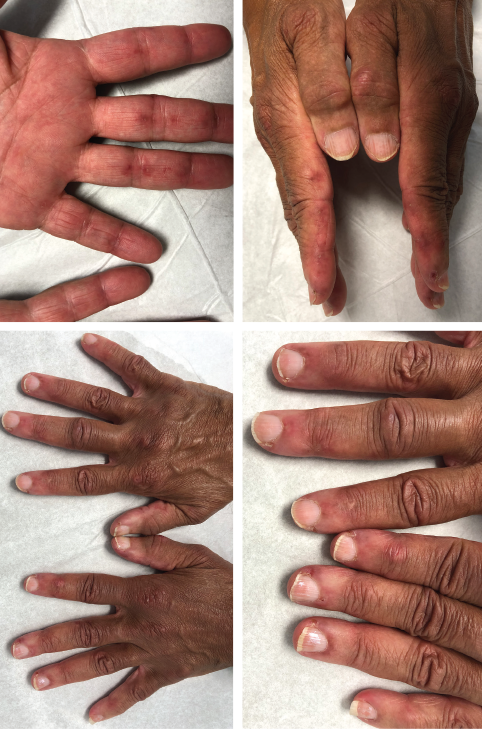
The initial physical examination was significant for a nonblanching, papular rash along the palmar aspects of the hands and digits, periungual erythema, and edema and tenderness of the proximal and distal interphalangeal joints of the hands.
The patient went to the emergency department multiple times for these symptoms, which improved with short courses of prednisone, but recurred each time after completion of treatment. A chest X-ray was consistent with hyperinflation of the lungs, with apparent scarring of the bases. A computed tomography scan (CT) of the abdomen and pelvis showed an 8 mm enhancing lesion in the right hepatic lobe, suggestive of a hemangioma, as well as streaky opacities in the bilateral lung bases. Autoimmune workup showed an erythrocyte sedimentation rate (ESR) of 28 mm/hour, no elevation of C-reactive protein, rheumatoid factor (RF) of 37.9 IU/mL, anti-cyclic citrullinated peptide (anti-CCP) antibody >250 u/mL, and undetectable anti-nuclear antibodies, anti-double stranded DNA, anti-Smith and anti-RNP antibodies (see Table 1, opposite).
At the time of the initial rheumatological evaluation, the patient remained significantly symptomatic, with persistent rash and severe hand and knee joint swelling and pain. Further workup showed elevated ESR of 45 mm/hour, normal creatinine kinase and aldolase levels, negative anti-Jo-1 antibody, and increased kappa and lambda light chains with preserved ratio and mixed glomerular and tubular proteinuria on serum and urine protein electrophoresis. X-rays of both hands showed sclerosis, lucencies of the lunates and degenerative changes without apparent erosion.
The patient was started on 200 mg of hydroxychloroquine daily, along with 40 mg of prednisone daily, for treatment of RA. Additional immunosuppressive therapy was held, pending further pulmonary and hematological investigations and age-appropriate cancer screening.
The concurrent manifestations of rash and rapidly declining pulmonary function without significant musculoskeletal symptoms increased our suspicion for overlap with CADM. A subsequent anti-melanoma differentiation-associated protein 5 (anti-MDA5) assay returned positive, which further supported this diagnosis.
An extensive workup was carried out to better characterize the disease and rule out possible concurrent systemic processes. Compared with the CT of the abdomen and pelvis performed one month before, chest CT images showed significant progression of her pulmonary disease, including diffuse nodular and coalescing opacities involving the lungs fairly symmetrically, from the pulmonary parenchyma, subpleural upper lobes, portions of the middle lobe and the lingula to the bibasilar regions. No suspicious or dominant nodules were apparent.
A pulmonary function test showed normal total lung capacity at 100%, and reduced carbon monoxide diffusing capacity at 64%. A ventilation-perfusion scan demonstrated a low likelihood for pulmonary embolism. A transthoracic echocardiogram showed preserved cardiac function, with left ventricular ejection fraction of 70% and no apparent pulmonary hypertension or valvular dysfunction.
Table 1: Laboratory Tests
Test Result Units Reference Interval
Erythrocyte sedimentation rate 28, increased to 45 mm/hr 0–40
C-reactive protein 0.8 mg/L 0.0–4.9
Creatinine kinase 142 U/L 24–173
Aldolase 8 U/L 3.3–10.3
Complement C3 102 mg/dL 82–167
Complement C4 31 mg/dL 14–44
Rheumatoid factor 37.9 IU/mL 0.0–13.9
Anti-cyclic citrullinated peptide antibodies >250 Units 0–19
Anti-melanoma differentiation-associated protein 5 antibodies Positive - Negative
Anti-nuclear antibodies Negative - Negative
Anti-double-stranded DNA antibodies <1 IU/mL <5
Anti-Smith antibodies <0.2 AI 0.0–0.9
Anti-Jo-1 antibodies <0.2 AI 0.0–0.9
Anti-chromatin antibodies 0.2 AI 0.0–0.9
RNP antibodies 0.3 AI 0.0–0.9
Anti-SSA antibodies <0.2 AI 0.0–0.9
Anti-SSB antibodies <0.2 AI 0.0–0.9
Anti-scleroderma-70 antibodies <0.2 AI 0.0–0.9
Anti-centromere B antibodies <0.2 AI 0.0–0.9
Anti-myeloperoxidase antibodies <9 U/mL 0.0–9.0
Anti-proteinase 3 antibodies <3.5 U/mL 0.0–3.5
Anti-cardiolipin IgG <9 GPL U/mL <15
Anti-cardiolipin IgM <9 MPL U/mL <13
Beta-2 glycoprotein I IgG <9 GPI IgG units 0–20
Beta-2 glycoprotein I IgM <9 GPI IgM units 0–32
Thyroid peroxidase antibody 31 IU/mL 0–34
Thyroglobulin antibody <1.0 IU/mL 0.0–0.9
Serum protein electrophoresis
Albumin 3.2 g/dL 2.9–4.4
Alpha-1-globulin 0.3 g/dL 0.0–0.4
Alpha-2-globulin 0.8 g/dL 0.4–1.0
Beta globulin 1.1 g/dL 0.7–1.3
Gamma globulin 1.7 g/dL 0.4–1.8
M-spike 0.1 g/dL Not observed
Globulin, total 3.9 g/dL 2.2–3.9
A/G ratio 0.8 0.7–1.7
Serum immunofixation
Immunoglobulin G 1547 mg/dL 700–1600
Immunoglobulin A 370 mg/dL 87–352
Immunoglobulin M 221 mg/dL 26–217
Immunofixation shows IgM monoclonal protein with lambda light chain specificity.
Bronchoscopy and lung biopsy showed nonspecific inflammation. Hematological and oncological investigations, including an unremarkable bone survey and negative colonoscopy and mammography, pointed to the diagnosis of monoclonal gammopathy of undetermined significance.
Taken all together, these findings were consistent with CADM with associated autoimmune ILD and no evidence of amyloidosis, infection or malignancy.
The patient was continued on 40 mg of prednisone daily and prophylactic trimethoprim and sulfamethoxazole; she was also started on 500 mg of mycophenolate mofetil (MMF) twice daily one month afterward (following the aforementioned testing), with the goal of titrating up to 3,000 mg daily for optimal control of her pulmonary disease.
Her shortness of breath, rash and synovial pain significantly improved on this regimen, but the rash and joint symptoms recurred with the tapering of prednisone. Thus, rituximab was initiated at 1,000 mg on days 1 and 15, and every six months thereafter, which has helped maintain adequate control of her joint and pulmonary symptoms.
At follow-up six months from the initial rheumatological evaluation, the patient continues to do well on this regimen, with successful taper of prednisone down to 5 mg daily, and discontinuation of prophylactic antibiotics.
Discussion
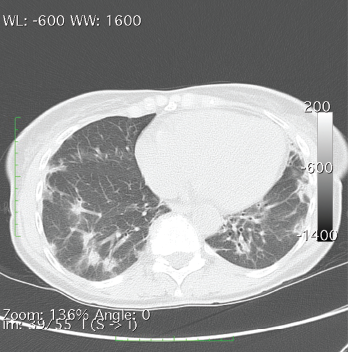
A CT of the chest without contrast showed multiple nodular and coalescing opacities involving the bilateral lungs diffusely, with more extensive involvement of the bibasilar regions.
Dermatomyositis encompasses a host of musculoskeletal and cutaneous symptoms, the latter of which are often heterogeneous in presentation. It is thought that differing serological responses, as reflected via various antibody markers, may represent differing immune responses, even unique phenotypes, with associated keratinocyte damage, which may explain the heterogeneity of presentation.4,5
MDA5, a receptor that binds with RNA and triggers a type I interferon response in infectious and inflammatory processes, was discovered in 2002 and has since become a point of interest in the study of autoimmune processes.6 Among patients with DM, certain features are strongly associated with anti-MDA5, including the absence of myositis and the presence of palmar papules, periungual, digital and elbow cutaneous ulcers, and rapidly progressive ILD, suggesting a unique phenotype that may pose its own treatment challenge.5
Initial treatment of DM often comprises anti-malarials, but there is no consensus on first-line therapy.3,7 Further, symptoms frequently require more aggressive treatment and a combination of multiple medications to achieve adequate control. Common additional agents include corticosteroids, calcineurin inhibitors (e.g., cyclosporine, tacrolimus), steroid-sparing immunosuppressants (e.g., methotrexate, azathioprine, cyclophosphamide, MMF) and intravenous immunoglobulin.8 Despite the use of these agents, symptoms often remain refractory.
Among patients with dermatomyositis, certain features are strongly associated with anti-MDA5, including the absence of myositis & the presence of palmar papules, periungual, digital & elbow cutaneous ulcers, & rapidly progressive ILD, suggesting a unique phenotype that may pose its own treatment challenge.
Recent advancements in biologics have introduced new treatment possibilities for DM. Therapies under investigation may be divided into those targeting B and T cells, and those targeting cytokines. Among these agents, rituximab, lenabasum, tofacitinib, apremilast, anakinra and tocilizumab have been promising in their effects on skin symptoms.3,9–13
In our patient with concurrent CADM and RA, agents that address both disease processes are of particular interest; the presence of ILD, common among CADM patients, adds further consideration to treatment choice. Rituximab, tofacitinib and tocilizumab, well studied in the treatment of RA, are of increasing interest in the treatment of CADM, given promising results in addressing skin symptoms.14
Regarding ILD, a suggested treatment approach is clinical evaluation and pulmonary function tests every three to six months, in addition to: 1) a combination of steroids and the choice of rituximab, cyclophosphamide or a calcineurin inhibitor for acute or severe pulmonary disease; or 2) a combination of steroids and the choice of MMF or azathioprine for chronic or milder ILD.15
These considerations ultimately led to our chosen treatment regimen of hydroxychloroquine, prednisone, MMF and rituximab.
In Sum
Given the scarcity of CADM, diversity of disease manifestation, refractory nature of the disease and possible overlap with other autoimmune or malignant processes, management of the disease remains difficult. A complicating factor is the absence of a firm consensus on treatment approach. However, investigations into the disease process and treatment options, particularly novel immunotherapy agents, continue to yield promising results and remain crucial in shaping our approach to the disease
going forward.
Vania Lin, MD, MPH, is an internal medicine resident at the University of Nevada, Reno, School of Medicine. She plans to pursue a fellowship in rheumatology.
Leah Krull, MD, is a practicing rheumatologist at Barton Memorial Hospital, South Lake Tahoe, Calif., and Carson Valley Medical Center, Gardnerville, Nev. She serves as adjunct faculty for the University of Nevada, Reno, School of Medicine.
References
- Galimberti F, Li Y, Fernandez AP. Clinically amyopathic dermatomyositis: Clinical features, response to medications and malignancy-associated risk factors in a specific tertiary-care-centre cohort. Br J Dermatol. 2016 Jan;174(1):158–164.
- Bendewald MJ, Wetter DA, Li X, Davis MDP. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: A population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010 Jan;146(1):26–30.
- Chen KL, Zeidi M, Werth VP. Recent advances in pharmacological treatments of adult dermatomyositis. Curr Rheumatol Rep. 2019 Aug 31;21(10):53.
- Sontheimer RD. Dermatomyositis: An overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002 Jul;
20(3):387–408. - Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): A retrospective study. J Am Acad Dermatol. 2011 Jul;65(1):25–34.
- Junior AGD, Sampaio NG, Rehwinkel J. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol. 2019 Jan;27(1):75–85.
- Callander J, Robson Y, Ingram J, Piguet V. Treatment of clinically amyopathic dermatomyositis in adults: A systematic review. Br J Dermatol. 2018;179(6):1248–1255.
- Pinard J, Femia AN, Roman M, et al. Systemic treatment for clinically amyopathic dermatomyositis at 4 tertiary care centers. JAMA Dermatol. 2019 Apr 1;155(4):494–496.
- Aggarwal R, Loganathan P, Koontz D, Qi Z, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatol (Oxford). 2017 Feb;56(2):247–254.
- Werth VP, Hejazi E, Pena SM, et al. FRI0470 A phase 2 study of safety and efficacy of lenabasum (JBT-101), a cannabinoid receptor type 2 agonist, in refractory skin-predominant dermatomyositis. Ann Rheum Dis. 2018;77(Suppl 2):763–764.
- Moghadam-Kia S, Charlton D, Aggarwal R, Oddis CV. Management of refractory cutaneous dermatomyositis: Potential role of Janus kinase inhibition with tofacitinib. Rheumatol (Oxford). 2019 Jun 1;58(6):1011–1015.
- Kurtzman DJB, Wright NA, Lin J, et al. Tofacitinib citrate for refractory cutaneous dermatomyositis: An alternative treatment. JAMA Dermatol. 2016 Aug 1;152(8):944–945.
- Kondo M, Murakawa Y, Matsumura T, et al. A case of overlap syndrome successfully treated with tocilizumab: A hopeful treatment strategy for refractory dermatomyositis? Rheumatol (Oxford). 2014 Oct;53(10):1907–1908.
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017 Jun 10;389(10086):2338–2348.
- Morisset J, Johnson C, Rich E, et al. Management of myositis-related interstitial lung disease. Chest. 2016 Nov;150(5):1118–1128.