- Rhabdomyolysis secondary to a viral infection;
- A toxin- or drug-induced myopathy;
- A metabolic myopathy; or
- An idiopathic inflammatory myopathy.
Aggressive intravenous hydration was initiated; however, his serum CK remained elevated. He developed oliguric acute renal failure secondary to pigment-induced, acute, tubular necrosis, which required hemodialysis. He also developed hypoxia due to heart failure from non-ischemic cardiomyopathy, with a left ventricular ejection fraction of 15–25%; this was followed by an episode of non-sustained ventricular tachycardia that required use of a personal defibrillator device.
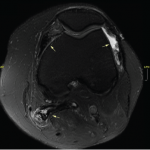
Magnetic resonance imaging of the thigh musculature showed extensively increased T2 signal throughout the gluteal and thigh muscles, which was interpreted as possibly consistent with an inflammatory myopathy (see Figure 2).
In the next evaluation step, a skeletal muscle biopsy was performed (see Figure 3). It revealed mildly increased variation in single fiber sizes, with few ring fibers. No inflammation was seen. (Note: These findings are non-specific and can be seen in muscular dystrophies, chronic myopathies or during myofiber regeneration). Electromyography showed non-specific myopathic changes. His urine toxicology screen was positive for cannabinoids, but otherwise unremarkable.
At this point, highest on the differential diagnosis was an undiagnosed myotonic dystrophy, possibly precipitated by stress from a viral illness, especially given that his mother also had cardiac muscle dysfunction. However, genetic testing for myotonic dystrophy was unrevealing. Additionally, genetic testing of both parents did not identify any abnormalities associated with myotonic dystrophy. Electron microscopy of the skeletal muscle biopsy was inconclusive. Tests for metabolic myopathies were also unremarkable.
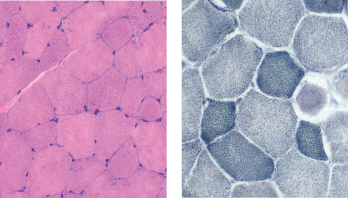
Figure 3: Muscle Biopsy. Hemotoxylin and eosin staining (left) and nicotinamide adenine dinucleotide staining (right) show mildly increased variation in single fiber sizes, with ring fibers.
Thyroid studies demonstrated a total T3 of 13 ng/dL (normal 70–204 ng/dL) and a low free T3 level of 1.1 pg/mL (normal 1.5-4.1 pg/mL). He was thought to have severe secondary hypothyroidism from inadequate, decreased peripheral conversion of T4 to T3 due to inadequate T4 supplementation, causing hypothyroid myopathy and precipitating rhabdomyolysis and cardiac muscle dysfunction.
He was started on T3 hormone supplementation with liothyronine (Cytomel). The next day, his serum CK level started improving, and within a week, his CK level decreased from 641,000 U/L to 17,000 U/L. The patient improved clinically as well, with resolution of his myalgias, dark urine and fatigue.
In a month, his renal function returned to normal, and he was able to stop hemodialysis. His cardiac function recovered, and a repeat echocardiogram demonstrated a systolic ejection fraction of 40–50%. The patient was able to resume his routine life and work. Repeat outpatient CK testing demonstrated a normal CK (81 U/L).
About a year later, he had another episode of rhabdomyolysis with a serum CK of 420,000 U/L, in the setting of non-adherence with liothyronine supplementation. He was restarted on liothyronine supplementation, again with resolution of his symptoms.