AMSTERDAM—Newly proposed systemic lupus erythematosus (SLE) classification criteria and new findings on SLE pathogenesis are two ways in which researchers and clinicians are getting a better grasp on the heterogeneous disease. The criteria and findings were discussed this June in a session at EULAR: the Annual European Congress of Rheumatology.
‘Paradigm Shift’ in SLE Classification
Sindhu Johnson, MD, PhD, director of the University of Toronto Scleroderma Program, unveiled new lupus criteria—supported, but not yet endorsed, by the ACR and EULAR. The process, largely designed to improve classification of early cases, was the most comprehensive for a set of lupus classification criteria and intended to produce “worldwide consensus,” Dr. Johnson said.
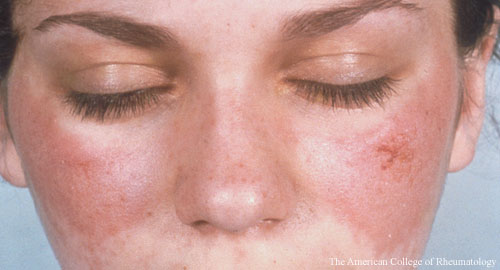
As entry criteria, the system includes an anti-nuclear antibody (ANA) titer of 1:80. From there, the criteria are grouped into seven clinical domains (constitutional, cutaneous, arthritis, neurologic, serositis, hematologic and renal) and three immunologic domains (antiphospholipid antibodies, complement proteins and highly specific antibodies). A score of 10 or more is enough to classify a patient as having SLE.
The criteria are weighted, with scores for each ranging from two for fever, etc., to 10, for Class III or IV lupus nephritis, meaning this criterion alone would be sufficient for a lupus classification.
According to Dr. Johnson, the process was designed to synthesize the collective knowledge of the world’s leading lupus clinicians into a usable format. “The clinical manifestations vary considerably,” she said. “Our ability to make a diagnosis is really based on the physician’s experience and education.”
The weighted score for each criterion was crafted through a painstaking process. Experts were faced with pairs of criteria and repeatedly chose which ones would most likely indicate a lupus classification. The system was validated using cases and controls submitted by international lupus centers. Cases and controls were retained only after agreement by three outside centers that they were in fact cases or controls.
Testing in the 1,001-patient derivation cohort produced a 98% sensitivity rate and a 97% specificity rate. The validation test, with a cohort of 1,270 patients, had 96.1% sensitivity and 94.4% specificity, which compares with 82.8% sensitivity and 93.4% specificity for the 1997 ACR classification criteria and 96.4% sensitivity and 84.5% specificity for the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria.
“We found very reasonable sensitivity and specificity, especially when compared against previous iterations of classification criteria,” Dr. Johnson said.
She said the criteria “represent a paradigm shift in the classification of SLE and provide a new foundation for SLE research.”