CPT II deficiency can be classified into three different presentations: 1) a lethal neonatal form involving multi-organ failure, 2) a severe infantile hepatocardiomuscular form, and 3) a milder, purely myopathic form. The severity of disease appears to be related to the type of mutation. Missense mutations that allow production of some partially functional enzyme activity are typically found in the milder myopathic form, while protein truncating mutations produce the more severe neonatal and infantile phenotypes.10
In contrast to the muscle glycogenoses, the myopathic form of CPT II deficiency often does not produce cramps, and muscle weakness should not be present between attacks. Serum CK levels are usually normal, as are EMG and muscle pathology. Patients may complain of muscle weakness and myalgia triggered by exercise, infection, or fasting. Other triggers include cold exposure and general anesthesia. Age of onset is typically in childhood but can vary widely. Diagnosis can be confirmed by whole blood DNA analysis that will detect known mutations in roughly 80% of patients.11 The plasma acylcarnitine profile serves as a screening test that will most often detect CPT II deficiency when the patient is symptomatic; however, functional enzyme analysis of fibroblasts or muscle tissue presumably offers higher sensitivity.10
Patients with CPT II deficiency should be instructed to avoid prolonged fasting (i.e., longer than 10 hours). Sustained, intensive exercise should be avoided. Carbohydrate loading prior to and during exercise may prevent attacks. We advocate dietary supplementation with medium-chain triglycerides, which provides an alternative substrate for fatty acid oxidation involving long-chain fatty acids.12
Mitochondrial Oxidative Phosphorylation Disorders
Defects of mitochondrial oxidative phosphorylation (mitochondrial disorders; respiratory chain disorders) are typified by multisystem involvement frequently involving muscle.1 Not infrequently, though, mitochondrial disorders manifest as isolated myopathies, as opposed to the encephalopathy and multisystem involvement associated with the classical infantile presentation. Late-onset mitochondrial myopathies feature proximal muscle weakness, easy fatigueability, and variably elevated CK levels. Lactic acidemia is often absent. Thus, mitochondrial myopathies are frequently misdiagnosed as inflammatory myositis upon presentation.
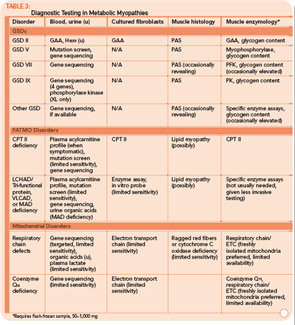
Classification by the underlying gene defect is complicated by tremendous genetic heterogeneity, with approximately 20% of mutations affecting mitochondrial DNA and the remainder affecting numerous nuclear genes. Utilizing the modified Walker criteria is essential to confirm the diagnosis of a mitochondrial myopathy because the presence of both characteristic clinical symptoms and diagnostic laboratory testing are required for confirmation of affected status.13 Muscle histology may feature ragged red fibers and decreased cytochrome C oxidase staining, or it may be normal. Functional enzymology on flash-frozen muscle tissue or fibroblasts allows quantitative assessment of electron transport chain (ETC; respiratory chain) complex activities. Flash-frozen muscle samples offer greater sensitivity than skin fibroblasts, but special handling required to maintain sample integrity is cumbersome and frequently delays or prevents the diagnosis. Noninvasive testing can be useful in some cases (see Table 3, p. 23, and Figure 1, p. 14).
Case 3
A 7-year-old male presented for inpatient consultation with recurrent vomiting, metabolic acidosis, a 6-month history of exercise intolerance accompanied by weakness, and poor weight gain. Physical examination revealed hepatomegaly and significant weakness of the lower extremities. Initial laboratory evaluation revealed significant metabolic acidosis, elevated plasma lactic acid (5 mmol/L; normal, 0.6–2.5), elevated serum CK (700 U/L; normal, 30–220), and decreased serum glucose (to 50 gm/dL). Urine organic acid analysis revealed elevated lactic acid and ketones. Plasma carnitine and acylcarnitine profiles were normal. Enzyme testing of cultured fibroblasts revealed normal carnitine palmitoyltransferase II activity and normal ETC complex II–IV activities. Subsequently, a muscle biopsy was performed, and histopathology revealed prominent ragged red fibers (see Figure 2A, p. 15). Electron microscopy revealed marked accumulation of abnormal mitochondria (see Figure 2B, p. 15). ETC testing of flash-frozen muscle confirmed a significant deficiency of complex III. Oral supplementation with coenzyme Q10 (120 mg), creatine monohydrate (3 gm), and alpha-lipoic acid (300 mg) twice daily was recommended, and gastrostomy tube feeding was initiated to provide adequate calories for growth. Within 6 months, the patient had recovered to his baseline level of activity and was gaining weight.
Mitochondrial Myopathies
Mitochondrial myopathies are emerging as a more frequent cause of metabolic myopathy than previously recognized. Classically, the mitochondrial myopathies were associated with point mutations in tRNA genes or large deletions involving the mitochondrial genome (e.g., myoclonic epilepsy and ragged red fibers or Kearns-Sayre syndrome). More recently, the deficiency of coenzyme Q10 synthesis has emerged as a cause for isolated myopathy.2 Interestingly, the underlying gene defect in cases of isolated myopathy has involved the electron transferring protein dehydrogenase (ETFDH) gene that was previously described in patients with multiple acyl-CoA dehydrogenase deficiency (MAD deficiency; glutaric acidemia type II).14 Previous reports attributed MAD deficiency to a “trifunctional protein deficiency,”1 creating confusion with a well-recognized trifunctional protein deficiency that affects three enzyme functions in FAO including LCHAD.10