TarikVision / shutterstock.com
We have all been to numerous lectures, grand rounds and other continuing medical education activities where the speaker, prepared and poised at the podium, begins his lecture with a title slide. Soon after, we see the ubiquitous conflicts of interest slide, which lists the invited speaker’s research funding, his consulting activities and his board memberships—all of which may influence the information presented. This slide is quickly clicked through, and the presenter then proceeds to deliver a well-researched, well-delivered lecture that is (usually) well received by the audience.
How does the audience truly know the presenter’s data and information are free from conflict? Is the conflict of interest slide adequate to protect the integrity of the information being delivered in any particular lecture or talk?
Recently, there has been no shortage of media reports pertaining to conflicts of interest in both the political and medical spheres. Despite the recent publicity, however, the concern over physician and researcher conflicts of interest is not new.
Definitions
When we speak about conflicts of interest, what do we really mean? The International Committee of Medical Journal Editors defines conflicts of interest as a type of conflict between a physician’s primary interest (i.e., patient care) and his secondary interests (e.g., financial gain, promotion).1 The concern is that one’s secondary interests may interfere or influence the primary interests.
Conflicts of interest can potentially erode the trust between the conflicted party and the audience. In a recent issue of the Journal of the American Medical Association, McCoy and Emanuel describe a three-part causal chain in which a physician has a secondary interest that has the potential to bias their judgment. If their judgment is influenced by their secondary interest, then the biased judgment could lead to some type of harm.2
This is why everyone, including professional medical societies, medical schools, medical journals and the lay public, should have a shared concern in recognizing, understanding and addressing the issue of conflict of interest.
Status Check
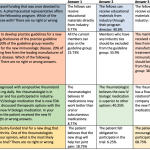
How are we doing at the ACR? A 2017 study examined the reporting of conflicts of interests during oral presentations at five different medical conferences, including the 2016 ACR/ARHP Annual Meeting.3 The ACR fared well when compared with the other academic meetings observed; 45 of the 45 presentations analyzed included some type of a conflict statement or a slide dedicated to conflicts of interest.
This study also looked at the duration of time the conflict of interest slides were left up for viewing. The median duration of time these slides were shown was approximately two seconds.
The ACR has well-defined policies regarding conflicts for speakers, volunteers, committee and board members. The ACR website includes the disclosures of all board members. Again, is disclosure enough to ensure that conflicts of interest can be adequately examined and assessed?
How Do We Know?
Determining whether a speaker’s judgment is biased is not necessarily straightforward. Although disclosures are an important part of identifying conflicts, some have argued that this measure is not sufficient.4,5 Critics argue that disclosing one’s relationships, financial or otherwise, does little in the way of alleviating concerns over conflicts of interest. In fact, they have argued that disclosures may, inevitably, complicate discussions pertaining to conflicts, as disclosures may be difficult to interpret by colleagues and patients. The difficulty in understanding disclosures could result in more skepticism and in loss of trust between the presenter and his or her audience.
Proponents of strict conflict of interest standards understand that levels of severity in conflicts exist and that not all conflicts of interest are created equal. It has been argued that severity of the conflict can be judged by two factors: 1) the likelihood a secondary interest will bias judgment and 2) the level of harm caused by the biased judgment.4
What means do we have available to manage and evaluate a speaker’s conflicts of interest? Disclosures can be an important tool in assessing conflicts, but as mentioned earlier, their effectiveness may be limited. Rather than simply listing sponsored activities on a slide, we should perhaps do our best to assess the degree of potential bias these activities may cause.4
More specifically, it is important to fully understand the financial gains a speaker may receive from sponsored activities. The social science and medical literature is rich with examples of how gifts, however small, influence one’s decision-making process.6 That said, there is a relative lack of research showing how exact dollar amounts influence the decision-making process. It has been hypothesized that the greater the financial interests, the greater the potential for bias and, thus, the greater the conflict of interest.2
Here are some of the potential ways the disclosure slide can be improved upon so the audience is better able to assess the speaker’s conflicts:
- In addition to listing the sponsored activities, the speaker should list the dollar amount received or expected from the activity and the manner in which these funds will be allocated. This clarification would allow the audience to assess the potential severity of the conflict rather than simply the activity itself.4
- The speaker should include the duration of time they have been involved in the particular activity or the duration of time they will receive funding.4
- The disclosure slide should be kept on the screen for a time sufficient for the audience to read it. Perhaps, the slide should be shown again at the conclusion of the presentation, because that would allow those audience members who were not present at the beginning to review the information.3
Ideally, as healthcare professionals, researchers and teachers, our primary interests should always be our focus. We must remain cognizant of the effect that secondary interests may have on our judgment regarding patient care, research and teaching. At times, our ability to recognize these potential clashes between our primary and secondary interests is skewed. It is, therefore, imperative that we remain as open and transparent as possible to ensure we remain committed to our ideals and don’t allow conflicts of interest to interfere with our ultimate goal: the care of patients with rheumatic diseases.
Athan N. Tiliakos, DO, is an assistant professor of medicine and the program director of the Rheumatology Fellowship Training Program at Emory University School of Medicine, Atlanta.
References
- International Committee of Medical Journal Editors. ICMJE definition.
- McCoy MS, Emanuel EJ. Why there are no “potential” conflicts of interest. JAMA. 2017 May 2;317(17):1721–1722.
- Grey A, Avenell A, Dalbeth N, et al. Reporting of conflicts of interest in oral presentations at medical conferences: A delegate-based prospective observational study. BMJ Open. 2017 Sep 21;7(9):e017019.
- Thompson DF. Understanding financial conflicts of interest. New Eng J Med. 1993 Aug 19;329(8):573–576.
- McKinney RE Jr., Pierce HH. Strategies for addressing a broader definition of conflicts of interest. JAMA. 2017 May2;317(17):1727–1728.
- Dana J, Lowenstein G. A social science perspective on gifts to physicians from industry. JAMA. 2003 Jul 9;290(2):252–255.
Editor’s note: Do you have an ethical dilemma you’d like to see discussed in this forum? Contact us via email at [email protected].