ChemoCentryx has developed TAVNEOS Connect, a patient support program designed to assist patients who are prescribed avacopan. To learn more about avacopan or TAVNEOS Connect, visit www.TAVNEOS.com.
ChemoCentryx is responsible for the discovery and development of TAVNEOS (avacopan) and owns the commercial rights to the drug in the United States. ChemoCentryx’s Kidney Health Alliance with Vifor Pharma provides Vifor Pharma with exclusive rights to commercialize TAVNEOS in markets outside the U.S.
TAVNEOS (avacopan) is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe following the European Medicines Agency (EMA) review is expected by the end of 2021.
INDICATION
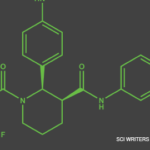
TAVNEOS (avacopan) is indicated as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. TAVNEOS does not eliminate glucocorticoid use.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Serious hypersensitivity to avacopan or to any of the excipients
WARNINGS AND PRECAUTIONS
Hepatotoxicity: Serious cases of hepatic injury have been observed in patients taking TAVNEOS, including life-threatening events. Obtain liver test panel before initiating TAVNEOS, every 4 weeks after start of therapy for six months and as clinically indicated thereafter. Monitor patients closely for hepatic adverse reactions, and consider pausing or discontinuing treatment as clinically indicated (refer to section 5.1 of the Prescribing Information). TAVNEOS is not recommended for patients with active, untreated and/or uncontrolled chronic liver disease (e.g., chronic active hepatitis B, untreated hepatitis C, uncontrolled autoimmune hepatitis) and cirrhosis. Consider the risk and benefit before administering this drug to a patient with liver disease.
Serious Hypersensitivity Reactions: Cases of angioedema occurred in a clinical trial, including one serious event requiring hospitalization. Discontinue immediately if angioedema occurs and manage accordingly. TAVNEOS must not be re-administered unless another cause has been established.
Hepatitis B Virus (HBV) Reactivation: Hepatitis B reactivation, including life threatening hepatitis B, was observed in the clinical program. Screen patients for HBV. For patients with evidence of prior infection, consult with physicians with expertise in HBV and monitor during TAVNEOS therapy and for six months following. If patients develop HBV reactivation, immediately discontinue TAVNEOS and concomitant therapies associated with HBV reactivation, and consult with experts before resuming.
Serious Infections: Serious infections, including fatal infections, have been reported in patients receiving TAVNEOS. The most common serious infections reported in TAVNEOS group were pneumonia and urinary tract infections. Avoid use of TAVNEOS in patients with active, serious infection, including localized infections. Consider the risks and benefits before initiating TAVNEOS in patients with chronic infection, at increased risk of infection or who have been to places where certain infections are common.