HORSHAM, PA—The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) has approved Tremfya (guselkumab) for adult patients with active psoriatic arthritis (PsA), a chronic progressive disease characterized by painful joints and skin inflammation.1,2 Tremfya is the first treatment approved for active PsA that selectively inhibits interleukin (IL) 23, a naturally occurring cytokine involved in normal inflammatory and immune responses associated with the symptoms of PsA.
The safety and efficacy of Tremfya in PsA have been demonstrated in two phase 3 clinical trials. Tremfya is administered as a 100 mg subcutaneous injection every eight weeks, following two starter doses at weeks 0 and 4. Tremfya can be used alone or in combination with a conventional disease-modifying anti-rheumatic drug (DMARD; e.g., methotrexate).
The Unmet Needs in PsA
PsA affects about 1.5 million Americans.3 Studies show that up to 30% of the more than 8 million Americans living with psoriasis will also develop PsA.4 There is currently no cure for the disease, and despite available treatments, many people living with PsA are still searching for more options that can help alleviate their symptoms and provide some relief.
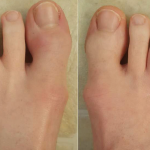
PsA is a chronic, progressive, immune-mediated disease characterized by joint inflammation, enthesitis (inflammation where the bone, tendon and ligament meet), dactylitis (severe inflammation of the digits of the hands and feet), axial disease (pain in the axial skeleton, primarily in the spine, hips and shoulders) and the skin lesions associated with psoriasis.A The disease commonly appears between the ages of 30 and 50, but can develop at any time.5 Though the exact cause of PsA is unknown, genes, the immune system and environmental factors are all believed to play a role in the onset of the disease. Without early recognition, diagnosis and treatment, the disease can continue to progress.5
“Psoriatic arthritis is a complex multi-faceted disease and, for many patients, additional biologic options are very much needed,” says Philip J. Measei, MD, DISCOVER-2 lead study investigator, director of rheumatology research at the Swedish Medical Center, Providence St. Joseph Health and Clinical Professor at the University of Washington School of Medicine, Seattle. “The two phase 3 pivotal trials evaluating the safety and efficacy of Tremfya, an IL-23 inhibitor, for the treatment of adults with active psoriatic arthritis provided insight into how it can improve joint symptoms. Today’s approval is exciting for both patients and their physicians, as there is now a new approach available to help manage the symptoms of active PsA that patients face day to day.”