A variety of mechanisms could explain the role of anti-dsDNA antibodies in promoting renal disease and their activity as nephrogenic or nephritogenic effector. Thus, these antibodies have the ability to bind to native glomerular structures as well as to endothelial cells. Once these immune complexes deposit in the kidney or penetrate into cells, they can act in the following ways:
- Modulate cell viability and induce apoptosis;
- Affect the proinflammatory pathways, up-regulating adhesion factors (ICAM, VCAM) in endothelial cells and IL-1, IL-8 mRNA expression in mononculear cells, and enhance IL-6 expression in glomerular mesangial cell; and
- Activate complement system via classical pathway leading to further tissue injury.
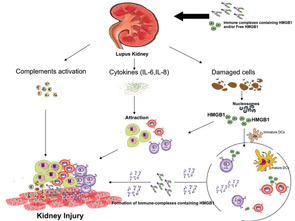
These effects are exerted directly by anti-dsDNA antibody via binding to Fc-receptor, which is present on the surface of many types of cell. In this situation, the receptor binds to the Fc region of antibody directly, or indirectly by DNA-containing immune complexes (via DNA antigen).16 Recently, it has been shown that these DNA-containing immune complexes also contain HMGB1, which is important for binding of these immune complexes to renal tissue and initiating renal injury in SLE patients.17
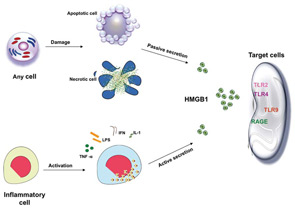
The potential role of HMGB1 in the pathogenesis of SLE is increased based on the fact that apoptotic cells accumulate in SLE and are the main source of autoantigens (nucleosomes) that may have bound HMGB1. Nucleosomes isolated form SLE sera were shown to be associated with HMGB1. Moreover, it has been shown that serum HMGB1 levels are increased in Chinese SLE patients compared with healthy controls and are correlated with disease activity and clinical parameters such as complements C3 and C4.18,19 In these studies, the investigators demonstrated that the elevation of HMGB1 levels was not associated with specific organ involvement. Also, Sato et al showed that sera of lupus nephritis patients didn’t show the tendency to be positive for HMGB1.13 However, in an IFN-accelerated murine model of SLE, administration of an anti-HMGB1 monoclonal antibody showed inhibition of proteinuria and led to kidney protection. These findings suggest that HMGB1 may play a role in the renal pathology. In contrast to previous studies on human SLE, our preliminary data show increased serum HMGB1 levels in active lupus nephritis patients.20 In addition, our data reveal a correlation of serum HMGB1 levels with proteinuria. The differences in the results of the various studies could reflect the 1) number and characteristics of patients included in the study; and 2) methods used to detect circulating HMGB1 (i.e., ELISA and Western blot).