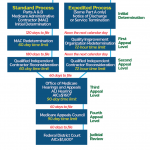
REFINEMENTS IN PERMANENT RAC PROGRAM
All new issues a RAC wishes to pursue for overpayments must be validated by CMS or an independent RAC validation contractor and posted to a RAC’s Web site before widespread review.
- Each RAC must hire a physician medical director as well as certified coders. A provider may request the credentials of the individuals making medical review determinations and request to speak to a RAC’s medical director regarding a claim denial.
- RACs cannot audit claims earlier than three years from the start of the program, with a maximum look-back date of October 1, 2007 (replacing a four-year look-back period in the demo).
- RACs are limited to requesting 10 medical records per 45 days from a solo physician, 20 medical records from a small practice of two to five physicians, 30 from a group of six to 15, and 50 from a large group of more than 16 physicians.
- RACs must send a detailed review results letter to providers following all complex reviews within 60 calendar days of the receipt of the medical records.
- RACs must pay back contingency fees when an improper payment determination is overturned at any level in the appeals process (demo RACs were allowed to retain them on determinations overturned on second- and third-level appeal).
- RACs must have in place by January 1, 2010 a Web-based claim status platform that will allow providers to track the status of medical record submissions to RACs.
- A RAC validation contractor will conduct a thirdparty review of RAC claim determinations and provide annual accuracy scores for each RAC.
Demo Program Lessons
In 2005, a RAC demonstration project was launched in California, Florida, and New York, states selected because they are the largest states in terms of Medicare utilization. The demonstration was expanded in 2007 to include Massachusetts, South Carolina, and Arizona.
Some physicians who were audited by a demo project RAC found the experience to be onerous. Some RACs demanded multiple physician repayments for claims that had previously been reviewed and adjudicated by a local Medicare carrier, misapplied codes, demanded hundreds of medical records from single practices, exceeded time limits for reviewing claims, interpreted clinical guidelines inappropriately, and applied new regulations retroactively to previously performed procedures, according to Michael Schweitz, MD, a rheumatologist from West Palm Beach, Fla., who was audited during the RAC demo program, and who testified before a Congressional committee last spring about RAC activities. “One six-physician practice was asked to pay back $100,000” in alleged overpayments, says Dr. Schweitz.