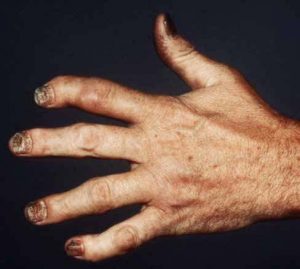
The ACR and the National Psoriasis Foundation (NPF) have published their first joint Guideline for the Treatment of Psoriatic Arthritis (PsA). The guideline, to be published in the January 2019 issues of Arthritis & Rheumatology, Arthritis Care & Research and the Journal of Psoriasis and Psoriatic Arthritis, will serve as an aid to practitioners managing active PsA in patients.1
[Editor’s note: The reference below was updated on Jan. 3, 2019, to reflect the print publication information.]
What the Guideline Includes
The management of active PsA in patients who are treatment naive and those who continue to have active PsA despite treatment is covered in the guideline, which includes strategies for optimizing therapy. The evidence-based guideline addresses use of oral, small molecule (OSM) drugs, tumor necrosis factor inhibitors (TNFi), interleukin (IL) 12/23 inhibitors, IL-17 inhibitors, CTLA4-Ig (abatacept) and a JAK inhibitor (tofacitinib).
Additionally, the guideline includes recommendations for management of patients with psoriatic spondylitis and predominant enthesitis, as well as treatment in the presence of concomitant inflammatory bowel disease (IBD), diabetes or serious infection. The presence of these concomitant conditions, according to the guideline, influences decisions about optimal therapy. Recommendations are included for a treat-to-target strategy, vaccinations and nonpharmacologic therapies. The intention is to help providers work with patients in selecting appropriate therapy in common clinical scenarios.
First-Line Therapy with TNF Inhibitor
Both the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) released recommendations for PsA in 2015.2,3 One significant difference in this new guideline from those developed by EULAR and GRAPPA: The recommendation to use a TNFi over an OSM agent (i.e., methotrexate) for patients who are treatment naive and have relatively active disease.
“The GRAPPA recommendations suggest that a physician bypass traditional DMARDs [disease-modifying anti-rheumatic drugs] and go to biologic agents in a case where there are bad prognostic signs. But this guideline recommends using anti-TNF agents first in people with active psoriatic arthritis,” says Dafna D. Gladman, MD, professor of medicine at the University of Toronto and Toronto Western Hospital, Ontario, and a member of the guideline’s core team.
“Many rheumatologists may already be doing what is recommended in the guideline, which will just confirm their actions,” she says. “But for some, the recommendation [will be surprising].”
“Methotrexate or other OSMs can be used first for people with less severe disease,” says Alexis Ogdie, MD, MSCE. Dr. Ogdie is assistant professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, and another member of the guideline’s core team.
The guideline notes that available, low-quality evidence is inconclusive regarding the efficacy of OSMs in the management of PsA. There is, however, moderate-quality evidence of the benefits of TNFi biologics, particularly for their impact on prevention of disease progression and joint damage.
One potential problem with this recommendation could be insurance coverage for use of a TNFi instead of methotrexate. Although the panel recognized that cost could be a factor, it “put considerations of quality of evidence for benefit over other considerations.”
Guideline Methodology
The guideline was developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, which rates the quality of the available evidence. Four teams selected by the ACR Quality of Care Committee worked on development:
- The Core Leadership Team supervised and coordinated the project;
- The Literature Review Team completed the literature search and abstraction;
- The Expert Panel, which included patients, patient advocates, rheumatologists, dermatologists, a dermatologist-rheumatologist and a rheumatology nurse practitioner, developed clinical questions (e.g., population, intervention, comparator outcomes [PICO]); and
- The Voting Panel, which included practitioners and patients.
A Patient Panel also reviewed the evidence and offered insights about values and preferences.
“Having the patient voice in every recommendation was important to the development of the guideline,” Dr. Ogdie says. “[The] separate Patient Panel gave us their input about the different recommendations. Most important, however, was the qualitative feedback they provided about their experiences with the different therapies. Bringing those to the table was really helpful in making some decisions.”
Patients outlined the negative effects of fatigue, nausea and malaise on their quality of life, and the potential risk for these side effects with certain therapies. They noted that some therapies could mean increased copayments, require additional travel time for more frequent appointments and increase adverse events.
The systematic literature review for guideline development spanned published English-language literature from the beginning of each database through mid-November 2016. Updated searches were completed on May 2, 2017, and March 8, 2018.
The Voting Panel, which included two patients, achieved consensus on the direction and strength of the recommendations included in the guideline. Six percent of the recommendations were strong, meaning the panel was confident the desirable effects of following the recommendation would outweigh the undesirable effects. The course of action for this type of recommendation should, therefore, apply to all or almost all patients. Strong recommendations were based on moderate- or high-quality evidence.
Ninety-four percent of the recommendations were conditional and were usually based on low- to very low-quality evidence. The guideline describes a conditional recommendation as one for which the desirable effects of following the recommendation probably outweigh the undesirable effects. The course of action would apply to a majority of patients, but a small proportion of clinicians and patients may not want to follow the recommendation. Conditional recommendations “are preference sensitive and always warrant a shared decision-making approach,” according to the guideline.
The guideline notes that even with the availability of new therapies for PsA, “there remains limited comparative efficacy/effectiveness evidence to inform treatment decisions. Conditional recommendations convey that, although the suggested course of action will be best for many patients there will be some patients in whom, considering their comorbidities and/or their values and preferences, the alternative represents the best choice.”
The high percentage of conditional recommendations “highlights how far we need to go for data generation for PsA,” Dr. Ogdie says. “We have limited data about how to treat the disease. We have a lot of data about individual drugs and how they compare to placebo, but that is not how we use drugs in clinical practice. We need more head-to-head drug trials to help us understand the best treatment strategies for patients.”
Treat-to-Target Strategy
The guideline supports a treat-to-target strategy, although the recommendation is conditional due to low-quality evidence. Optimal disease management using a targeted approach can possibly avoid or delay irreversible joint damage, associated functional limitations, joint deformities and disability associated with PsA. The Voting Panel did not address specific targets to be recommended or used, but stated that a treatment target would likely use either minimal disease activity or disease activity in PsA.
A different target “may be chosen through patient-provider discussion,” according to the guideline. Not using treat-to-target can be considered in patients when concerns about the higher frequency and/or severity of adverse events, higher cost of therapy or higher patient burden of medication with tighter control exist, according to the guideline.
Highlighted Recommendations
In addition to the recommendation about first-line use of a TNFi in patients with relatively active disease, several other recommendations are highlighted:
- Patients with active PsA despite treatment with an OSM should switch to a TNFi biologic over a different OSM;
- An OSM may be chosen over a TNFi biologic if the patient has mild to moderate disease, has a preference for oral over parenteral therapy or has concerns about the adverse effects of a biologic;
- A TNFi biologic would not be a good choice for those with congestive heart failure, previous serious or recurrent infections or demyelinating disease; and
- For patients who have active PsA and concomitant IBD despite treatment with an OSM:
- Switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic, which is ineffective for IBD;
- Switch to a TNFi monoclonal antibody biologic over an IL-17i biologic, which is ineffective for IBD; and
- Switch to an IL-12/23i biologic over switching to an IL-17i biologic.
Nonpharmacologic Interventions
The guideline makes several recommendations for nonpharmacologic interventions. One strong recommendation supported by moderate-quality evidence is for smoking avoidance/cessation. This recommendation was based on evidence linking smoking to a reduced efficacy of biologics, the recognized benefits of smoking cessation and the well-established links of smoking with mortality, cancers, and heart and lung diseases in the general population.
Physical therapy, occupational therapy, massage therapy and acupuncture as tolerated are recommended for most patients, along with weight loss for patients who are overweight or obese.
“There is good information available about how weight loss is helpful to patients in multiple domains. The TNFi can work better and patients do better when they lose weight. This is important, but it is often underappreciated,” Dr. Ogdie says.
The complete guideline is available online on both the ACR website and the NPF website.
Kathy Holliman, MEd, has been a medical writer and editor since 1997.
References
- Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019 Jan;71(1):5–32.
- Gossec L, Smolen JS, Ramior S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016;68:1060–1071.