vitstudio / shutterstock.com
Imagine telling a patient “You’re in remission!” and finding out it’s not true. The last thing you want to do is get it wrong clinically and put your patient on an emotional rollercoaster.
With large vessel vasculitis (LVV) in particular, physicians struggle to be accurate, to determine if indeed the disease has gone away or just gone into hiding.
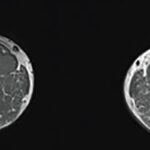
Peter Grayson, MD, MSc, is head of the Vasculitis Translational Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (NIH) in Bethesda, Md. Dr. Grayson and colleagues published an article in the March 2018 issue of Arthritis & Rheumatology on the use of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) as an imaging biomarker for large vessel vasculitis.1
“Like many rheumatologists,” he says, “I have had a patient with LVV who, according to the bloodwork, was in remission, but in fact was not. Determining whether the disease is still active is easier to do shortly after the initial diagnosis when things are more obvious, but it becomes more difficult over the disease course. Tests, such as the erythrocyte sedimentation rate, are not necessarily reliable to determine disease activity.
“There are fewer clinical trials in LVV [than for] forms of small vessel vasculitis, in part because there are no validated outcome measures of disease activity in LVV. “It’s hard to determine if a therapy is effective when it is simultaneously challenging to figure out if the disease is still active.”
‘FDG-PET was useful in differentiating patients with clinically active LVV from controls (a sensitivity of 85% & a specificity of 83%). However, we also observed that the majority of patients with LVV in clinical remission at the time of imaging had FDG-PET scan findings that looked like ongoing vasculitis.’ —Dr. Grayson
Does Bloodwork = Guesswork?

Dr. Grayson
“There is always the concern with LVV that medications improve the blood markers of inflammation without improving inflammation at the tissue level,” Dr. Grayson says. “In fact, when autopsies have been performed on patients with LVV who died of other causes and were thought to be in clinical remission at the time of death, ongoing active vasculitis is almost always detected in the arteries. So somehow, it appears the treatments for LVV are not getting at the underlying vascular inflammation.
“FDG-PET is traditionally used to detect metabolic activity in cancer cells,” he says. “However, it has been known for years that FDG-PET can also be used to visualize inflammation.”
Dr. Grayson goes on to explain, “Inflammatory cells concentrate in FDG, meaning that FDG-PET might be useful to assess the degree of inflammation present in a patient’s tissues in certain diseases.
“At the NIH,” he says, “we created a vascular imaging study in LVV. In this study, detailed clinical assessment is linked to advanced molecular imaging. This study includes the initial results from our cohort. Other groups have studied the role of FDG-PET to detect vascular inflammation in LVV, [but] almost all of these studies have been retrospective and focused on the time of initial diagnosis. In our study, we prospectively imaged patients with LVV over the course of disease at regular intervals. We also clinically evaluated all the patients the day prior to imaging so that we were blinded to the imaging results.”
The Details
The researchers performed 170 FDG-PET scans in 115 individuals. The 56 patients with LVV included those from the two major LVV categories: giant cell arteritis and Takayasu’s arteritis. The 59 controls were patients with hyperlipidemia, patients with diseases that mimic LVV and healthy people. All patients underwent a clinical evaluation; patients with LVV were imaged at six-month intervals. The team used a score based on global arterial FDG uptake, the PET Vascular Activity Score (PETVAS), to examine associations between activity on the PET scan and clinical characteristics and to predict relapse.
Dr. Grayson says, “FDG-PET was useful in differentiating patients with clinically active LVV from controls (a sensitivity of 85% and a specificity of 83%). However, we also observed that the majority of patients with LVV in clinical remission at the time of imaging had FDG-PET scan findings that looked like ongoing vasculitis.”
What does this tell us? “We rarely have histology to compare to the imaging studies,” says Dr. Grayson, “so we are always speculating about the source of FDG arterial uptake. Is it ongoing active inflammation, metabolic activity from damage/repair processes or secondary atherosclerosis? The results from our study provide indirect support to the idea that arterial FDG uptake during clinical remission is likely driven by a combination of these factors. We found that severe FDG uptake during remission predicted clinical relapse within an average of 15 months of follow up.”
What Helps? Who Gets Better?
“Our study was conducted within an observational cohort, which is prone to certain forms of bias,” Dr. Grayson says. “A great next step would be to incorporate imaging studies into randomized controlled trials in LVV to see how treatment modifies imaging signals in the arteries.
“What’s especially interesting—and what we want to explore going forward—is which medications improve both clinical and imaging-based assessments of disease activity.
“This study is hopefully just the tip of the iceberg,” he says. “The next phases of the project will be to standardize quantitative analysis of FDG-PET in LVV, to discover blood and tissue-based biomarkers associated with clinical vs. imaging-based assessment, and to develop validated imaging-based outcome measures for use in clinical trials in LVV.”
Dr. Grayson’s message to his colleagues? “We know that if you image patients later in the course of the disease, you may well find discordant results between the clinical and lab assessments. What this discrepancy means in terms of long-term management remains to be seen. It may be premature to base treatment decisions on imaging findings alone. Ultimately, we need complementary prospective studies in other cohorts before we really know if FDG-PET activity during remission predicts worse long-term clinical and angiographic outcomes. We will also need to balance benefits of advanced imaging in LVV with potential risks, including radiation exposure and cost.
“The bottom line,” says Dr. Grayson, “is that clinical, serologic and imaging-based assessments of disease activity provide complementary and unique information in LVV. We need to learn how best to incorporate these modalities into clinical practices.”
Elizabeth Hofheinz, MPH, MEd, is a freelance medical editor and writer based in the greater New Orleans area.
Reference
- Grayson PC, Alehashemi S, Bagheri AA, et al. 18F-fluorodeoxyglucose–positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol. 2018 Mar;70(3):439–449.