Enzyme Inhibitors
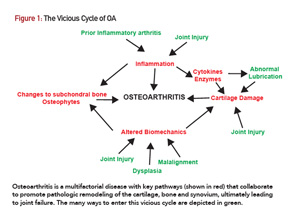
The concerted effort of proteases is thought to mediate the slow degradation of articular cartilage extracellular matrix observed in OA.8 These proteases include zinc-dependent enzymes, such as MMP13 and the aggrecanases (e.g., ADAMTS5), as well as cysteine proteases, such as cathepsin K.
Type II collagen and aggrecan are the major targets of these enzymes, along with other matrix components that maintain cartilage integrity. Mice with genetic mutations in these enzymes have been studied in post-traumatic OA models. For example, ADAMTS5-deficient mice are protected from OA that ensues after joint instability surgery, a model that recapitulates the post-traumatic degenerative process.9,10 Similar results have been obtained with MMP13- and cathepsin K-deficient mice.11,12
Conversely, mice with elevated activity of these enzymes, either through genetic overexpression or deletion of endogenous protease inhibitors, display accelerated OA.8 Specific chemical inhibitors of these proteases have shown promising results in mouse models. Inhibitors of ADAMTS5, MMP13 and cathepsin K all reduce histologic manifestations of post-traumatic OA in animals.12-15 Some early phase trials were carried out in humans with inhibitors against two of these enzymes (e.g., aggrecanase and cathepsin K; clinicaltrials.gov record locators NCT00427687 and NCT00397683). However, the results of these studies were never published, and there has been no action on this front for a few years, perhaps because of toxicity concerns or a lack of predictive biomarkers and intermediate outcome measures in OA. The possibility of locally delivering these drugs into OA joints could renew interest as a way to maximize the benefit to risk ratio.13
An alternative method to target the zinc-dependent catabolic enzymes was revealed in a recent paper in the journal, Cell.16 The authors found elevated levels of zinc in OA cartilage from mice and humans. Inflammatory stimuli, such as interleukin-1, induced expression of a potent zinc transporter to increase local levels. Unexpectedly, the influx of zinc induced expression of MMPs and ADAMTS5. Over-expression of the zinc transporter produced OA-like lesions, and genetic ablation prevented post-traumatic OA. These data suggest that zinc is a key mediator of catabolic protease expression in OA, in addition to its established role as a cofactor for these enzymes. It will be very interesting to see whether this pathway can be leveraged for the treatment of OA, perhaps by zinc chelation or inhibition of zinc transport.