For almost 30 years, low-dose methotrexate has been the therapy for rheumatoid arthritis (RA) to which every other therapy is compared. Indeed, methotrexate forms the base upon which therapy for RA is built; even when the patient does not achieve a sufficient therapeutic effect from methotrexate, new agents are added to, rather than substituted for, this agent. It is now clear from registration studies on the treatment of early RA that methotrexate is nearly as effective as biological agents and that the combination of methotrexate and a biologic agent is significantly better than either alone. It can be truly said that the use of methotrexate to treat RA has transformed rheumatology as a specialty and raised therapeutic expectations for our patients and, for pharmaceutical companies developing new therapeutic agents, raised the bar for registration of new drugs. Surprisingly, methotrexate was added to the formulary for the treatment of RA without a solid understanding of how the drug suppressed inflammation.
When methotrexate was first introduced for the treatment of cancer in the 1940s, it was one of the first products of intelligent drug design. Methotrexate was designed to be a close analog of folic acid and to block the folate-dependent steps in de novo purine and pyrimidine biosynthesis. Despite the high doses (grams/week used in the therapy of some malignancies) of methotrexate required to inhibit cellular proliferation, methotrexate was assumed to have the same mechanism when initially introduced to treat RA in the 1970s and 1980s. Thus, when methotrexate was first added to the therapeutic armamentarium, it was assumed that the mechanism by which methotrexate diminished inflammation and disease activity in RA was inhibition of purine and pyrimidine synthesis, thereby killing or preventing the proliferation of the lymphocytes and other cells thought to be responsible for the inflammation of RA.
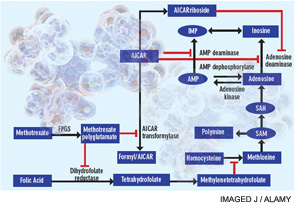
A cytotoxic or antiproliferative effect of methotrexate is clearly consistent with its known actions in the treatment of malignancies; however, methotrexate-mediated cytotoxicity cannot fully explain the antiinflammatory actions of methotrexate. Perhaps the most telling point in this regard is that, unlike the antiproliferative effects of methotrexate, neither folic acid nor folinic acid (except when given at the same time as methotrexate) consistently reduces or reverses the antiinflammatory effects of methotrexate in RA in randomized, blinded prospective trials.1 Moreover, the development of leukopenia, common in patients receiving high doses of methotrexate to treat malignancies, is a sign to cut back the dose or stop the use of methotrexate in treating patients with RA. Thus, other explanations for methotrexate’s mechanism of action have been explored (see Figure 1).
Figure 1
Proposed mechanisms of the antiinflammatory actions of methotrexate. Methotrexate exerts its antiinflammatory actions through a number of cellular mechanisms. Competitive inhibition of dihydrofolate reductase diminishes the de novo synthesis of purines and pyrimidines by preventing the regeneration from dihydrofolate of tetrahydrofolate, which is essential for the generation of folate cofactors required for purine and pyrimidine synthesis. Reduction of the levels of methyl donors, such as tetrahydrofolate and methyltetrahydrofolate, by the inhibition of dihydrofolate reductase results in the inhibition of the generation of lymphotoxic polyamines through methionine and SAM. Inhibition of AiCAr transformylase results in an increase in intracellular AiCAr levels. This increase has potent inhibitory effects on AMP deaminase and adenosine deaminase, which are involved in the catabolism of AMP and adenosine to IMP and inosine, respectively. The consequent accumulation of adenosine confers antiinflammatory effects. Levels of AiCArriboside, a metabolite of AiCAr, also accumulate and inhibit adenosine deaminase.