Experts presented ways to rethink journal club to improve engagement and how an image-based program can help teach the assessment of cutaneous lupus erythematosus across differing skin tones.


Experts presented ways to rethink journal club to improve engagement and how an image-based program can help teach the assessment of cutaneous lupus erythematosus across differing skin tones.

No one-size-fits-all approach exists for the care and treatment of patients with systemic sclerosis (SSc) and SSc with pulmonary involvement. Here, experts discuss some best clinical practices for these patients.

Linda Childers |
Jennifer May, MD, a rheumatologist with Rapid City Medical Center, South Dakota, completed her undergraduate degree at Augustana University, Sioux Falls, S.D., and earned her medical degree at the University of South Dakota School of Medicine, Vermillion. She was in the fourth grade when she first began playing the viola. Although she came to love…

Gretchen Henkel |
Medal for Excellence Awarded to Graciela Alarcón, MD Graciela (Chela) S. Alarcón, MD, MPH, is the emeritus Jane Knight Lowe Chair of Medicine in Rheumatology at the University of Alabama at Birmingham (UAB), and a professor of medicine (emeritus) at Universidad Peruana Cayetano Heredia (UPCH), Lima, Perú, her alma mater. Last fall, she received the…
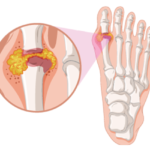
Although the diagnosis and treatment of gout are sometimes straightforward, practitioners encounter challenges in patients with atypical presentations, as well as those with medically complex situations or refractory disease. Here, gout experts share insights into some of these scenarios. Flare in Hospitalized Patients When not contraindicated, the 2020 ACR Guideline for the Management of Gout…
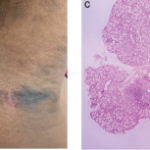
Marina Barguil Macedo, MD, MSc; Featured Image from Latin America & the Caribbean |
Lichenoid Cutaneous Lupus Erythematosus Mimicking Acanthosis Nigricans The photos depict a 45-year-old woman who presented to the Lupus Clinic of the University of São Paulo, Brazil, with lesions closely resembling acanthosis nigricans on her neck (A and B). The lesions had been present for four months. The patient had lived with systemic lupus erythematosus (SLE)…

Charmayne Dunlop-Thomas, MS, MPH, Nancy Delnay, MSN, APRN-CNP, & the ARP Research Subcommittee |
Information overload generated by the media, family, friends and colleagues is apparent today. Personal beliefs play an important role in how we filter and process the abundant information available and subsequently identify its utility in daily life. Regardless of professional specialty, individual beliefs underpin personal approaches to clinical care, research development and engagement with patients…
James T. Rosenbaum, MD,* Shravani Mikkilineni, MD, MBA, Hadi Khazaei, MD, Davin C. Ashraf, MD, & John D. Ng, MD, MS, FACS |
When my daughter was a second-year internal medicine resident at Massachusetts General Hospital, Boston, she called me excitedly one evening. “Dad,” she reported, “I think I saw the optic nerve for the first time today with an ophthalmoscope.” I suppose I should have shared her exuberance, except that when I went to medical school, a…
Samantha C. Shapiro, MD |
A 25-year-old Mexican American woman with a five-year history of systemic lupus erythematosus (SLE) presents with refractory, acute cutaneous lupus erythematosus (ACLE) and subacute cutaneous lupus erythematosus (SCLE) affecting the scalp, face and hands. Her serologic phenotype is characterized by elevated anti-nuclear, anti-double-stranded deoxyribonucleic acid (dsDNA), anti-ribonucleoprotein (RNP), anti-Smith and anti-SS-A (Ro) antibodies and chronically…
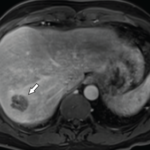
Katherine Chakrabarti, MD, & Andrew Vreede, MD |
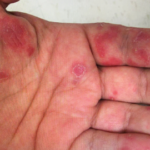
Abscesses are typically caused by infections, but some are, instead, sterile. Aseptic abscesses (AAs) are characterized by the same neutrophil-rich histopathology as infectious abscesses; however, they don’t improve with antibiotics. Rather, AAs require treatment with anti-inflammatory medications. Although relatively rare, this phenomenon is important for rheumatologists to recognize given its frequent association with underlying systemic…