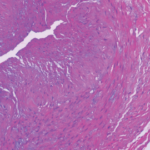
Giant cell arteritis (GCA) is a granulomatous vasculitis of large- and medium-sized arteries, usually affecting the cranial branches of the aortic arch. It is the most common vasculitis, with the highest risk factor being age. Accurate diagnosis and prompt initiation of therapy are of great importance to prevent serious complications, with the most feared being…