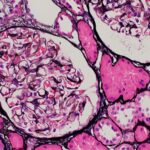
In this study, Fava et al. investigated longitudinal autoantibody profiles in a racially diverse cohort of patients with lupus nephritis to define noninvasive serological biomarkers of histologic class and one-year treatment response to standard of care. In addition, the researchers determined how these biomarkers changed over time to provide further insights into treatment response and…