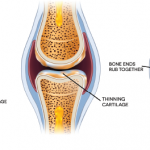
AMSTERDAM—Early identification and treatment of rheumatoid arthritis (RA) are two of the most pressing concerns in the field, an expert said at EULAR: the Annual European Congress of Rheumatology. He described the latest efforts to identify patients at risk of RA development and insights on quick referral to rheumatologists. Karim Raza, BM, BCh, PhD, Arthritis…