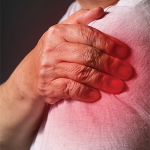
WASHINGTON, D.C.—For patients with osteoarthritis and other age-related musculoskeletal diseases, treatment with mesenchymal stem cells may soon offer a potent way to slow and repair degenerative signs of disease. This is the goal, a goal that is moving from the laboratory to the clinic as results from ongoing randomized clinical trials show the safety and…