 A recent study challenges the long-held practice of starting symptomatic pulmonary sarcoidosis patients on corticosteroids and points to a greater role for rheumatologists in its treatment.
A recent study challenges the long-held practice of starting symptomatic pulmonary sarcoidosis patients on corticosteroids and points to a greater role for rheumatologists in its treatment.
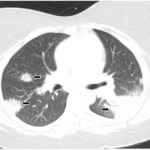
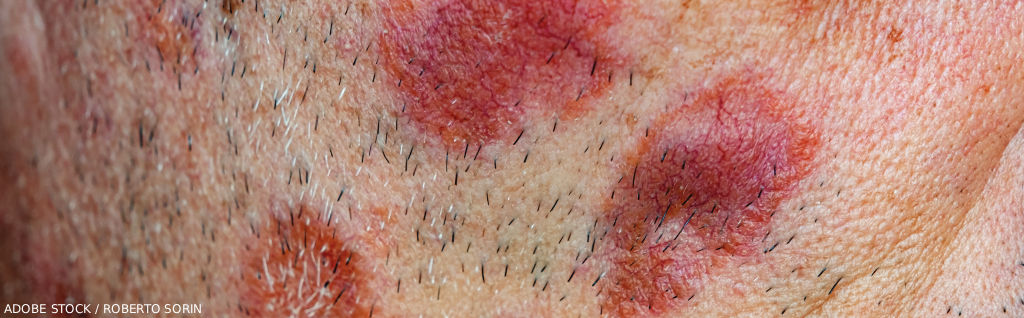
In general, sarcoidosis involves development of granulomas in various parts of the body. These lumps or nodules are composed of white blood cells and surrounded by fibrous tissue. Depending on their location and size, granulomas can turn into fibrosis, causing permanent scarring. The lungs are the most involved organs, but others—including the skin, eyes, heart and liver—may also be affected. Guidelines issued by the European Respiratory Society (ERS) in 2021 recommend prednisone as the first-line treatment for pulmonary sarcoidosis, but this suggestion is based on low-quality evidence in the absence of well-designed trials.1
In a recent multicenter, open-label trial involving patients with previously untreated pulmonary sarcoidosis, researchers in The Netherlands show that initial treatment with the antimetabolite methotrexate is as effective as the corticosteroid prednisone in changing lung function, as measured by the percentage of the forced vital capacity (FVC). Types of adverse events differed somewhat between patients on prednisone and methotrexate. However, the percentage of patients who experienced adverse events was similar in both groups, according to the Effectiveness of Methotrexate Versus Prednisone as First-line Therapy for Pulmonary Sarcoidosis (PREDMETH) trial.2
“The main message from PREDMETH is that methotrexate can, and should, be considered a real alternative to prednisone for patients with pulmonary sarcoidosis,” says senior author Marlies Wijsenbeek-Lourens, MD, PhD, a pulmonologist and head of the Center for Interstitial Lung Diseases and Sarcoidosis, Erasmus Medical Center, Rotterdam. “Patient education and shared decision making are key, so together with the patient we can choose the treatment that best fits their situation.”
Because sarcoidosis can affect multiple organs, Dr. Wijsenbeek-Lourens emphasizes that no single specialty can cover all aspects of care. “We need each other’s expertise,” she notes.
Arthritis or bone involvement can significantly affect patients’ quality of life and require targeted rheumatologic treatment.
“Rheumatologists also bring valuable experience with long-term immunosuppressive therapy and monitoring of side effects, which can help pulmonologists optimize treatment strategies,” Dr. Wijsenbeek-Lourens adds. “Close interdisciplinary collaboration is not just helpful, but increasingly essential to deliver the best care for our patients.”
PREDMETH
Prednisone has long been the cornerstone of treatment for pulmonary sarcoidosis. But it has troublesome side effects, including weight gain, sleep disorders, psychological problems and increased cardiovascular risk. Sarcoidosis treatment guidelines issued in 1999 by the American Thoracic Society and the European Respiratory Society established corticosteroids as a first-line treatment, but pointed to the need for less toxic alternatives, notes Dr. Wijsenbeek-Lourens.3