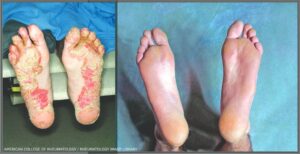
Reactive Arthritis: Keratoderma blennorrhagicum on both feet before (left) and after treatment with a TNFα antagonist. (Click to enlarge.)
Reactive arthritis is a sterile inflammatory arthritis that often occurs in the weeks following a gastrointestinal or genitourinary tract infection. A form of spondyloarthritis, it can present with myriad extra-articular features and has a variable, sometimes protracted, course that may warrant long-term immunosuppression.
Due to varied presentations, numerous inciting organisms and a lack of specific biomarkers, reactive arthritis is challenging to categorize and study intensively.1 Therefore, management recommendations are often derived from case series and case reports.
In this review, we explore the epidemiology, clinical manifestations, management and prognosis of reactive arthritis, with insights and pearls from experts in the realm of spondyloarthritis.
Historical Context
The combination of arthritis and gastrointestinal or genitourinary symptoms has long been recognized but poorly understood. Arthritis and urethritis were first described together in the 16th century, although identification of culprit organisms, such as chlamydia, didn’t occur until nearly three centuries later.2
In 1916, French physicians Noel Fiessinger and Edgar Leroy described a “syndrome conjunctivo-ureto-synovial” that had occurred in four soldiers after an outbreak of dysentery.3 Four days later, Hans Conrad Julias Reiter described a similar illness, characterized by the triad of arthritis, conjunctivitis and non-gonococcal urethritis. Initially called Reiter’s syndrome, the condition was renamed reactive arthritis given Reiter’s allegiance to the Nazi Party in the 1930s, along with the recognition that a majority of patients do not present with the triad of signs and symptoms he described.4
Epidemiology
The incidence of reactive arthritis varies widely in outbreak studies, but is generally low, around 1% or less, and reported at a global frequency of 0.3–30 per 10,000.5,6 It commonly presents in adults 20–40 years old, with an equal sex distribution; however, reactive arthritis due to Chlamydia trachomatis is more common in men.7
The incidence of HLA-B27 positivity varies widely, with either a small or no increase in frequency reported in outbreak studies and epidemiological surveys, but up to 60–80% reported in hospital-based studies.8,9 HLA-B27 positivity is known to predict a more severe and chronic course, as well as the presence of more extra-articular manifestations. 10 HLA-B27 is, therefore, considered a prognostic tool more than a marker of susceptibility.11
A family history of spondyloarthritis is considered an additional risk factor for chronicity and joint damage.12