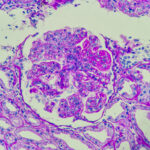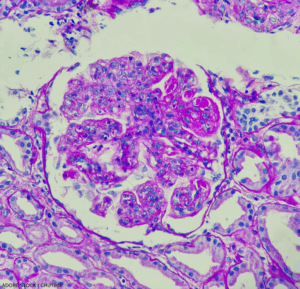
Lupus nephritis, showing wire loop and hyaline thrombi, PAS stain, magnification 400x, photo under microscope.
At a session of ACR Convergence 2024, speakers shared key elements of the new guideline on the screening, treatment and overall management of lupus nephritis in children and adults.1 This guideline attempts to balance the risks of medication side effects with the important goal of preserving kidney function.
This is the ACR’s first lupus guideline since 2012. At that time, recommendations were made for induction therapy with high-dose glucocorticoids plus immunosuppressant medications, such as mycophenolate mofetil (MMF) or cyclophosphamide, with MMF often used as a maintenance therapy.2
Since then, more information has become available regarding additional effective agents, and the U.S. Food & Drug Administration has approved both belimumab (a B cell inhibitor) and voclosporin (a calcineurin inhibitor) for the treatment of lupus nephritis. Although rates have been declining, up to 20% of lupus nephritis patients have faced end-stage renal disease within the first decade of diagnosis. New therapies and therapeutic strategies may provide better results.3
This guideline follows the established ACR guideline development format. It is based on a systematic litereature review with input from a patient panel, adult and pediatric rheumatologists and nephrologists, and a rheumatology physician assistant.
Screening & Biopsy
In patients with lupus but without known kidney disease, screening for proteinuria every six to 12 months is a strong recommendation. It’s also strongly recommended that patients with systemic lupus erythematosus (SLE) receive a proteinuria screening if they experience a disease flare in another organ system.
The guideline also emphasizes the importance of prompt kidney biopsy if lupus nephritis is suspected, which can help facilitate timely treatment. Biopsy in the case of proteinuria greater than 0.5g/g or other signs of unexplained impaired kidney function are conditional recommendations.
Additionally, a repeat kidney biopsy is conditionally recommended in patients previously in lupus nephritis remission who present with suspected flare. Kidney biopsy is also recommended for patients who’ve had appropriate treatment but ongoing signs of disease, such as ongoing proteinuria, hematuria or decreased kidney function.
Lisa R. Sammaritano, MD, a professor of clinical medicine at Weill Cornell Medicine and Hospital for Special Surgery, New York, and the lead author on the guideline, noted that clinicians should not wait for biopsy results to give glucocorticoids if lupus nephritis is suspected.
Triple Therapy Treatment
A key element of the guideline is its emphasis on triple therapy for new onset or flaring lupus nephritis. In this context, triple therapy consists of glucocorticoids (i.e., high-dose, pulse intravenous [IV] glucocorticoids followed by an oral taper) given with two additional immunosuppressive therapies.