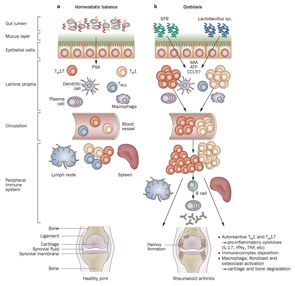
Among environmental risk factors studied in relationship with RA development, the most notable examples include hormones, smoking, alcohol, and infection. Multiple viruses and bacteria have been associated with disease incidence but, to date, causation could not be established. Given the central role of host–microbe interactions, massively parallel DNA-sequence analyses of human microbiomes now offer the potential for important new insights relevant to autoimmune processes. One of the more intriguing environmental covariates modulating autoimmunity involves bidirectional crosstalk between the host and the oral and intestinal microbiomes (see Figure 1).
Figure 1
Host–microbiota interactions in health and inflammatory arthritis. A: In healthy individuals, a well balanced host–microbial cross-talk is essential for the maintenance of homeostasis. A thick mucus layer and epithelial cells prevent direct contact with the gut-associated immune cells, which constantly survey the contents of the intestinal lumen and eliminate undesired antigens. Commensal bacteria, such as Bacteroides fragilis, can activate pro tolerogenic machinery. A specific cell wall component, PSA, is sufficient to induce TREG-cell activation, IL-10 production, and TH17-cell repression to avoid uncontrolled inflammation. B: When either genetic or environmental factors alter the balance in the microbiota composition, dysbiosis ensues. Potentially harmful micro-organisms (such as SFB or Lactobacillus) predominate and local expansion of proinflammatory cells (TH17 cells, TH1 cells and others) occurs via different molecules (such as ATP, SAA, or CCL5 signaling). These autoreactive T cells migrate to peripheral immune compartments and activate B cells to differentiate into autoantibody-producing plasma cells. These cells and antibodies then migrate to synovial tissue where the inflammatory cascade is amplified through the activation of effector components, including macrophages, fibroblasts, osteoclasts, cytokines, and proteinases. If self-perpetuating, this process can lead to arthritis and pannus formation.
Abbreviations: ATP, adenosine-5′-triphosphate; CCL5, CC-chemokine ligand 5; IFNγ, interferon γ; IL-17, interleukin-17; PSA, polysaccharide A; SAA, serum amyloid A; SFB, segmented filamentous bacteria; TH1, type 1 T helper cell; TH17, type 17 T helper cell; TREG, regulatory T cell.
Source: Nat Rev Rheumatol. 2011;7:569-578. Reproduced with permission of Nature Pub. Group.
The roots for this appealing notion can be traced many centuries back. In the 1600s, and in the midst of the Great Plague, a devoted and simple Dutch tradesman named Antony van Leeuwenhoek managed to see beyond the tangible. His innovation was a combination of magnifying lenses and metallic skeletons which gave birth to a peculiar creature that would modify science forever: the microscope. With this instrument, van Leeuwenhoek made his most fascinating discovery. Diminutive forms of life, invisible to the bare eye, populated our body cavities and most surfaces on earth.