Dr. Nigrovic introduces the review with a case study. He next discusses the hallmarks of MAS, which include severe systemic inflammation, usually with fever, together with elevated ferritin, a low platelet count and other markers of disseminated intravascular coagulation and transaminase elevation. Dr. Nigrovic reviews the latest data on the pathogenesis, diagnosis, treatment and prognosis of MAS. He discusses MAS of the central nervous system and Still’s disease-associated lung disease.
 The review includes informative tables, detailed images and legends, and many references.
The review includes informative tables, detailed images and legends, and many references.
Case Study
The case study followed a 17-year-old male patient who presented with a three-week history of daily fever, spiking in the afternoons, along with a transient rash and arthralgias. Physical examination revealed a body temperature of 38.5ºC, a palpable spleen tip, and a macular and linear erythematous rash on his trunk.
Laboratory tests showed a white blood cell count of 15,000/μL, hematocrit at 37%, platelets at 453,000/μL, an erythrocyte sedimentation rate (ESR) of 83 mm/h, C-reactive protein (CRP) at 8 mg/dL, and ferritin at 850 ng/mL. Aspartate transaminase (AST) and alanine transaminase (ALT) were normal. IL-18 was 36,000 pg/mL (normal <800 pg/mL) and CXCL9 was 230 pg/mL (normal <121 pg/mL).
While awaiting prior authorization for treatment with the recombinant IL-1 receptor antagonist anakinra, the patient received naproxen. His fevers somewhat improved, occurring every few days for two weeks, but then worsened. Re-evaluation of the patient revealed the following: body temperature 40ºC, hematocrit 31%, platelets 230,000/μL, ESR 45 mm/h, CRP 16 mg/dL, ferritin 43,000 ng/mL, AST 450 U/L, and ALT 430 U/L. IL-18 increased to 180,000 pg/mL and CXCL9 increased to 3,200 pg/mL.
The patient’s symptoms improved after treatment with pulse glucocorticoids and anakinra, and he was transitioned to the anti-IL-1β antibody canakinumab. He remains in clinical remission off glucocorticoids, with normal CRP, ferritin and CXCL9, an IL-18 of 8,300 pg/mL.
Diagnosis & Treatment
An unusually direct connection exists “between the pathogenesis of MAS and the strategies used to diagnose and treat it,” Dr. Nigrovic writes. He highlights insights gained from primary hemophagocytic lymphohistiocytosis (HLH), “a set of rare inborn errors of immunity that present as MAS in infancy or early childhood, often from defects in cell-cell killing mediated by perforin, a protein expressed by CD8+ T cells, natural killer cells and other lineages.”
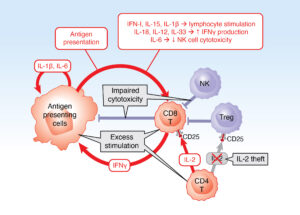
The vicious cycle of macrophage activation syndrome. Antigen-presenting cells (macrophages and dendritic cells) and lymphocytes (primarily CD8+ T cells) form a cytokine-generating vicious cycle driven by multiple activation pathways (red ar-rows). Under normal conditions, this positive feedback loop is stopped by inhibito-ry pathways (blue), including killing of activated antigen-presenting cells and lym-phocytes, regulatory T cells and elimination of triggers, such as viruses (not shown). In MAS, factors leading to uncontrolled activation of this vicious cycle (gray boxes) include genetic and acquired defects in cell-cell killing, excess stimula-tion (Still’s disease activity, infection, malignancy) and IL-2 theft by highly activated CD8+ T cells expressing the high-affinity IL-2 receptor α chain CD25.
