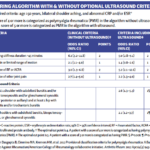
A minority of patients with polymyalgia rheumatica (PMR) who were new to rheumatology practice were prescribed steroid-sparing agents through two years of follow-up. This is according to a large, U.S.-based cohort study, published in Arthritis Care & Research, which also found that nearly two-thirds of the patients remained on glucocorticoids beyond one year.1 “Our study…