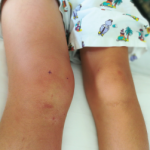
ATLANTA—Managing pediatric patients with rheumatic disease involves special considerations, such as developmental concerns and physiological traits that may affect dosing of medications, according to two experts. During a session at the 2019 ACR/ARP Annual Meeting, Courtney Kremer, ARNP, a pediatric nurse practitioner at the University of Iowa Stead Family Children’s Hospital, Iowa City, and Jessica…