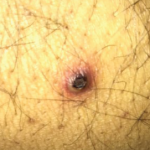
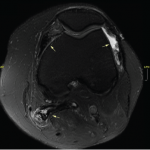
As a veteran rheumatologist, I remember the clinical trials of etanercept’s (Enbrel’s) efficacy. And when the drug was first approved in 1998, I participated in those clinical trials and realized the effectiveness was astonishing. It was easy to tell which patients were treated with etanercept vs. those who received placebo, even though both groups were…