1. A 68-year-old new female patient has an appointment to see the rheumatologist in four days. The patient has her medical records sent over from her primary care physician, neurologist and endocrinologist for the rheumatologist to review prior to the visit. Upon receipt, the rheumatologist spends 55 minutes reviewing the records and making notes. Can…
Search results for: infliximab

Long-Term Benefits, Risks of Biologic Disease-Modifying Anti-Rheumatic Drugs in Patients with RA
Two decades have passed since the first biologic disease-modifying anti-rheumatic drug (bDMARD) was approved. Studies on the long-term use of biologics in different disease states, such as for cardiovascular disease (CVD) and malignancy, as well as for knee/hip replacement, reveal some encouraging news. In clinical trials, bDMARDs have been shown to increase the risk of…

The ACR Lobbies Against New Part B Drug Cost Adjustment Rule
The ACR and a number of other physician medical associations are lobbying for an immediate legislative fix to a piece of the MACRA law that factors high-cost Part B drugs into a rheumatology practice’s Medicare reimbursement rate through the Merit-Based Incentive Payment System (MIPS). This change, which goes into effect immediately, will impact practices in…
Biosimilars Great Debate: To Switch or Not?
SAN DIEGO—Should patients with rheumatic diseases switch from a biologic to its biosimilar? At the 2017 ACR/ARHP Annual Meeting’s Great Debate, held Nov. 5, two rheumatologists argued whether to switch or stay put based on safety, efficacy and potential cost savings. First to the podium to make the case for switching, Jonathan Kay, MD, tweaked…

Physical Activity, Exercise Can Benefit Patients with RA
While medical advances in rheumatoid arthritis (RA) have led to improvements in disease control and quality of life for patients worldwide, the rate for stable remission remains low.1 Management of RA symptoms is traditionally accomplished through a combination of medications and nonpharmacological interventions.2 This approach can prevent the development of secondary adverse health outcomes. Two…

Unwise Choices: EHRs, PBMs, Drug Costs Are Leading to Physician Burnout
My dear electronic health records How do I dislike thee? Let me count the ways Adaptation of Sonnet 43 By Elizabeth Barrett Browning, 1806–1861 As my tenure as physician editor winds down, it’s worth reviewing some of the more nettlesome issues confronting clinicians that have been previously discussed in these pages and gauge their current…

Report on EU’s Experience with Biosimilar Drugs Released: Will U.S. Experience Be Similar?
As questions about biosimilar medications swirl among U.S. rheumatologists, a recently released report sheds some light on the European experience with biosimilars—and may offer some important insights for the U.S. market. The report, Biosimilars in the EU: Information Guide for Healthcare Professionals, was released in late April by the European Medicines Agency (EMA) and the…
CMS Implements Part B Modifiers for Biosimilars
With the advent of biosimilars to the marketplace, the Centers for Medicare and Medicaid Services (CMS) now requires modifiers to identify the manufacturer of a biosimilar/biological product on Part B claims. Modifiers were put in place to provide the CMS with the necessary data needed to track claims payments, as well as the ability to…
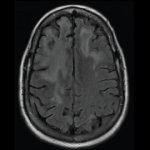
Fellows’ Forum Case Report: Progressive Multifocal Leukoencephalopathy and RA
Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with erosive destruction of diarthrodial joints. Patients who are seropositive are more prone to developing extra-articular manifestations, such as rheumatoid lung, rheumatoid nodules and others. With the development of disease-modifying anti-rheumatic drugs (DMARDs), the incidence and severity of these extra-articular manifestations has declined. Below, we describe…

Anti-TNF-Alpha Agents May Improve Endothelial Function Patients with RA
A systematic review has found that anti-tumor necrosis factor alpha treatment may improve endothelial function in RA patients. Despite the heterogeneity of the included studies, a random-effects model showed a significant improvement in endothelial function in this patient population after receiving infliximab, adalimumab or etanercept…
- « Previous Page
- 1
- …
- 19
- 20
- 21
- 22
- 23
- …
- 42
- Next Page »