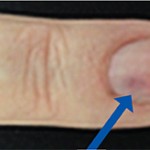
Ethical issues that arise in the average rheumatology practice and in clinical research are often straightforward. The AMA Code of Medical Ethics and the Office Practice and Procedures Manual offer useful information.1 In research, the Protocol and Investigators Agreement spells out who you can enroll and how the trial must be conducted. But still—even when…