(Reuters)—President Barack Obama’s nominee to head the U.S. Food and Drug Administration defended his ties to the pharmaceutical industry on Tuesday during a Senate committee hearing that included questions on soaring drug prices. Presidential candidate Bernie Sanders was among the Democrats who grilled Dr. Robert Califf (64), who joined the FDA in January as a…
Signatures to Be Filed for California Drug Price Referendum
SACRAMENTO, Calif. (Reuters)—Backers of a referendum aimed at reducing the cost of prescription drugs in California said Wednesday that they had gathered more than enough signatures to place their measure on the November 2016 ballot in the most populous U.S. state. The measure by the AIDS Healthcare Foundation would require the state to pay no…
Don’t Bank on U.S. Drug Price Rises, Warns GSK Boss
LONDON (Reuters)—Pharmaceutical companies cannot depend on ever increasing prices in the U.S. and will need to find a new balance between incentives for innovation and access to medicines, according to the chief executive of GlaxoSmithKline. High drug prices have come under fierce political fire recently in America, the industry’s biggest and most profitable market, with…

FDA Issues Stronger Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Warning
The U.S. Food and Drug Administration (FDA) has toughened the existing warnings for nonsteroidal anti-inflammatory drugs (NSAIDs) due to their stroke and myocardial infarction (MI) risk increase.1 Due to a continual review of these products, FDA is requiring label updates for all prescription NSAIDs. Over-the-counter (OTC) NSAIDs already list the increased risk of MI and…
Transatlantic Divide: How U.S. Pays Three Times More for Drugs
LONDON (Reuters)—U.S. prices for the world’s 20 top-selling medicines are, on average, three times higher than in Britain, according to an analysis carried out for Reuters. The finding underscores a transatlantic gulf between the price of treatments for a range of diseases and follows demands for lower drug costs in America from industry critics such…
Drug Industry Must Address Image Problem over Prices
NEW YORK (Reuters)—Teva Pharmaceutical Industries Ltd’s research chief said on Tuesday the drug industry must act responsibly when it comes to pricing medications, given the mounting anger over the high cost of therapies in the U.S. Democratic presidential candidate Hillary Clinton brought new attention to the issue last week, proposing to cap patients’ treatment costs,…

FDA Issues Warning for Joint Pain from Diabetes Drugs
Severe and disabling joint pain has been connected to the use of dipeptidyl peptidase-4 inhibitors and combination therapies for diabetes, prompting a new FDA warning…
Alcohol Use Complicates Chronic Disease Management in Teens
NEW YORK (Reuters Health)—High school students with chronic medical conditions who drink alcohol are more likely than their nondrinking peers to forget or skip taking their medications, according to an online survey. “I was surprised to see such a clear association between alcohol use and medication nonadherence—a finding which really brings home the need to…
FDA Proposes Adding Suffixes to Distinguish Biosimilar Drug Names
WASHINGTON (Reuters)—The U.S. Food and Drug Administration proposed on Thursday identifying cheaper versions of biologic drugs with a suffix to distinguish them from their more expensive, branded counterparts. The FDA said its draft guidance is designed to prevent the inadvertent substitution of non-interchangeable products and to make it easier to monitor and track usage once…
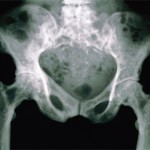
The ACR’s State-of-the-Art Clinical Symposium: Rheumatologists Weigh in on Tough-to-Treat Cases, Paget’s Disease, Imaging
CHICAGO—A 49-year-old woman has had RA for eight years. She has a rheumatoid factor reading of 35, an aCCP reading of 160, erythrocyte sedimentation rate of 42, plus erosions. She has been on methotrexate. She tried etanercept for six months, but then it stopped working. She was on 40 mg of adalimumab weekly, but it…
- « Previous Page
- 1
- …
- 4
- 5
- 6
- 7
- 8
- …
- 21
- Next Page »