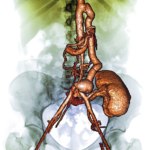
A small study shows baricitinib effectively treats refractory Takayasu arteritis & allows for glucocorticoid reduction with no serious adverse events recorded.

Katie Robinson |
A small study shows baricitinib effectively treats refractory Takayasu arteritis & allows for glucocorticoid reduction with no serious adverse events recorded.
Using data from registries across Europe and Canada, Aymon et al. compared the incidence of major adverse cardiac events (MACE) in patients with RA treated with JAK inhibitors, TNF inhibitors or biologic DMARDs with other modes of action over two years. The researchers did not find an increased risk of MACE with JAK inhibitors over that time frame.

Experts provided an in-depth review of Janus kinase (JAK) inhibitors, discussing the latest research on their use in the treatment of specific rheumatic conditions, the risks associated with them and more.
Deborah Levenson |
Results of the international SELECT-GCA study suggest that upadacitinib may be an effective new oral treatment for giant cell arteritis.

What Are the Therapeutic Alternatives When a Janus Kinase Inhibitor Fails to Work? SAN DIEGO—Treatment alternatives after Janus kinase (JAK) inhibitor failure in real-life conditions were analyzed and presented at ACR Convergence 2023 by Pablo Francisco Muñoz Martínez, a rheumatologist at the Hospital Universitario y Politécnico La Fe, Sagunto, Spain.1 JAK inhibitors are newer, targeted…

Research from Choi et al. provides insights into the risk of infection in patients with rheumatoid arthritis (RA), comparing patients treated with Janus kinase inhibitors vs. tumor necrosis inhibitors. The most frequent infection was herpes zoster, with patients treated with JAK inhibitors having a significantly greater risk of herpes zoster infection than those treated with TNF inhibitors.
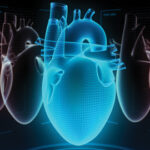
Research by Chicre et al. found that Janus kinase inhibitors may significantly increase the risk of major adverse cardiac events and all-cause death in patients with rheumatoid arthritis (RA) when compared with other RA treatments. This study highlights the need for more comparative safety studies.

ACR Convergence 2021—On Nov. 5, Karen H. Costenbader, MD, MPH, professor of medicine, Harvard Medical School, and director of the lupus program, Brigham and Women’s Hospital, Boston, gave a whirlwind review of the most important clinical rheumatology publications of the past year. Testing New Medications for Rheumatic Disease ADVOCATE Trial of Avacopan Dr. Costenbader first…
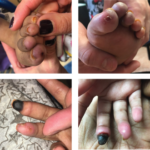
Marta Michalska-Smith, MD, & Colleen K. Correll, MD, MPH |
Janus kinase 1 and 2 inhibitors (jakinibs) have been approved for the treatment of rheumatoid arthritis, psoriatic arthritis and, most recently, juvenile idiopathic arthritis. They have also shown promise in the treatment of interferon (IFN) mediated diseases. The Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway is the principal signaling pathway for…

In a study, Janus kinase inhibitors proved effective for patients with difficult-to-treat rheumatoid arthritis (RA).