NEW YORK (Reuters Health)—The investigational drug romosozumab led to gains in hip bone mineral density (BMD) that were not seen with teriparatide in older women with osteoporosis transitioning from bisphosphonate therapy in the STRUCTURE study. Amgen’s romosozumab is a monoclonal antibody that inhibits sclerosin, a negative regulator of bone formation. In addition to stimulating bone…
Search results for: postmenopausal osteoporosis
New Bisphosphonate Therapy Recommendations for Postmenopausal Osteoporosis
A task force of the American Society for Bone and Mineral Research (ASBMR) has released new recommendations delineating the potential benefits and risks of prolonged therapy with oral and IV bisphosphonate therapy and providing guidance on duration of bisphosphonate therapy for postmenopausal osteoporosis.1 The task force makes clear that data and clinical experience on which…
Combination Therapy Bests Monotherapy in Severe Postmenopausal Osteoporosis
NEW YORK (Reuters Health)—The combination of denosumab and teriparatide improves bone microarchitecture and estimated strength more than either drug alone in women with severe postmenopausal osteoporosis, researchers have found. Dr. Joy Tsai, from Massachusetts General Hospital in Boston, and colleagues conducted a single-site, two-year, open-label, randomized controlled trial involving 94 women aged 45 or older…
FDA Greenlights Osteoporosis Drug for Postmenopausal Women
(Reuters)—The U.S. Food and Drug Administration says it has approved Amgen’s osteoporosis treatment for postmenopausal women who are at high risk of fracture. Evenity (romosozumab-aqqg), developed jointly with Belgium-based UCB SA, helps reduce the risk of fracture by increasing bone mass and mildly inhibiting the break down of bone minerals. Romosozumab-aqqg belongs to a new…

To the Bone: Experts Discuss Developments in Osteoporosis Care
During ACR Convergence 2023, experts discussed developments in the treatment and diagnosis of osteoporosis, addressing risks of treatment discontinuation, the use of bone turnover markers in patient assessment and vitamin D.
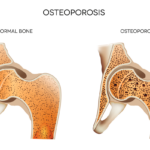
ACR Releases Update to Glucocorticoid-Induced Osteoporosis Guideline
The ACR recently released an update on the prevention and treatment of glucocorticoid-induced osteoporosis.1 The guideline, which includes information on the new therapies abaloparatide and romosozumab, emphasizes the importance of shared decision making by patients and clinicians, and also gives information on the importance of sequential therapy after stopping certain osteoporotic prevention therapies. Fracture Prevention…

FDA Approves Abaloparatide to Treat Men with Osteoporosis & a High Risk of Fracture
In late December, the FDA approved subcutaneous abaloparatide for the treatment of men with osteoporosis at a high risk of fracture. This approval is based on a placebo-controlled study that showed abaloparatide led to significant increases in bone mineral density of the lumbar spine, total hip and femoral neck. Abaloparatide was approved in April 2017 for the treatment of postmenopausal women with osteoporosis at high risk for fracture.

Zoledronic Acid vs. Oral Bisphosphonates: Osteoporosis Treatments & the Risk of Developing Osteonecrosis of the Jaw
A study from Amigues et al. found that bisphosphonate-associated osteonecrosis of the jaw is rare in patients with osteoporosis and may occur more often in patients treated with injectable zoledronic acid than in those treated with the oral bisphosphonates.

Challenging Cases in Osteoporosis: Tips from an Expert
Using three complicated patient cases, Kenneth G. Saag, MD, MSc, shared his expertise on osteoporosis and walked through his thought process and the literature, during a session of the 2022 ACR Education Exchange.

Osteoporosis Experts Discuss Bisphosphonate Holidays
ACR CONVERGENCE 2020—Bisphosphonates are an important treatment for millions of older Americans with osteoporosis because the drugs inhibit osteoclastic bone resorption to reduce the risk of painful, debilitating fractures.1 More than 20 years ago, data emerged that bisphosphonates have a long terminal half-life.2 So after years of therapy, could some patients take a drug holiday?…
- 1
- 2
- 3
- …
- 14
- Next Page »