In August, Abbvie submitted a new drug application for upadacitinib to treat ankylosing spondylitis. And in September, the FDA approved an oral solution of tramadol hydrochloride for pain.


In August, Abbvie submitted a new drug application for upadacitinib to treat ankylosing spondylitis. And in September, the FDA approved an oral solution of tramadol hydrochloride for pain.

In its first phase 3 clinical trial, intravenous tramadol has met its primary endpoint for relieving postoperative pain…

New studies show ABT-494 is an effective alternative to anti-TNF alpha agents, as well as methotrexate, for RA. Also, the FDA issued a warning against using tramadol for young patients due to their increased risk of respiratory side effects…

Mohammad A. Ursani, MD, FACP, RhMSUS, Iman Qaiser, MD, & Mamdouh Mahmoud, PhD |
Intermittent fasting—defined as alternating between cycles of eating and going without food over a given period of time—has become popular with individuals seeking to lose weight or balance their lifestyle in recent years. During Ramadan (a period based on the Gregorian calendar that changes from year to year), able-bodied Muslims are obligated to observe a…
Michael Cammarata, MD |
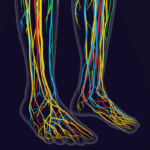
Small fiber neuropathy is a common form of peripheral neuropathy with multiple potential etiologies and a varied clinical presentation. It can’t be detected by nerve conduction studies, making it an elusive and often overlooked entity. Small fiber neuropathy is well documented in several rheumatic diseases, and its symptom burden can profoundly affect quality of life….

Guy Katz, MD, Joanna Marco, MD, & Jean Liew, MD, MS |
One of the most fun events at every annual meeting is the ACR Knowledge Bowl. In this quiz show-style trivia game, fellows form teams to compete for top prizes. There’s a charismatic host, a panel of esteemed judges and an auditorium packed with cheering spectators. Beyond the fun of competing against your peers from other…

Oshrat E. Tayer-Shifman, MD; Kimberley Yuen, BSc, MD; Zahi Touma, MD, PhD, FACP, FACR; William Daniel Soulsby, MD; Aleksandra Kostic, BSE; Valia Leifer, MA; & Elena Losina, PhD, MSC |
MoCA as a Screening Test in SLE Assessing the utility of the Montreal Cognitive Assessment (MoCA) By Oshrat E. Tayer-Shifman, MD, Kimberley Yuen, BSc, MD, & Zahi Touma, MD, PhD, FACP, FACR Why was this study done? Cognitive impairment is a common manifestation of systemic lupus erythematosus (SLE), with a prevalence of 40% based on…
Vania Lin, MD, MPH, Rebecca Johnson, MD, & Lisa Suter, MD |
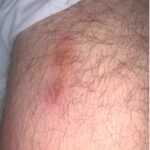
Polyarteritis nodosa (PAN) is a necrotizing vasculitis, predominantly involving medium-sized arteries, that causes systemic disease, and, less commonly, cutaneous-limited disease. The population prevalence for PAN ranges from 2 to 33 per million.1-3 Estimates vary due to the increased recognition and classification of other forms of vasculitides over time and variation in the regional prevalence of…

Cyltezo (adalimumab-adbm) may be interchanged for Humira (adalimumab) for all indications, according to an October decison by the FDA. The FDA has also approved a new combination of celecoxib and tramadol for pain management.

Acute gout can be very painful, causing patients to seek treatment in the emergency department. A retrospective study of pain interventions for gout in Rhode Island found that nearly 30% of patients received prescriptions for opioid medications over 30 months. Of these prescriptions, over 80% were for patients who had never been exposed to opioids…