The Case
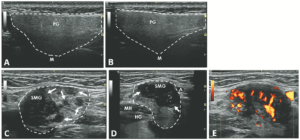
FIGURE 1: Normal longitudinal left (A) and right (B) parotid glands. (C) Left longitudinal submandibular gland with nodular hypoechoic lesions. (D) Right longitudinal submandibular gland with larger hypoechoic lesions producing a bulging on the surface of the gland. (E) Large amount of Doppler signal within the right submandibular gland. Parotid gland (PG), submandibular gland (SMG), masseter muscle (M), mylohyoid muscle (MH) hyoglossus muscle, facial artery (A). The white arrows indicate areas of IgG4 involvement. The white dashed lines outline the glands. (Click to enlarge.)
A 47-year-old woman presented with a one-year history of bilateral submandibular gland swelling, mild symptoms of xerostomia and xerophthalmia and arthralgias in her fingers. A review of systems was otherwise unremarkable.
On physical examination, her submandibular glands on both sides were enlarged and had a firm texture. Her parotid glands were normal, as were her cervical, axillary and inguinal lymph nodes. The conjunctiva was normal, and the oral mucosa was moist. The rest of the exam was normal.
An ultrasound revealed hypoechoic nodules replacing large segments of both submandibular glands, with distortion of gland architecture, creating irregular gland surfaces and increased Doppler signal bilaterally. The parotid glands appeared normal (see Figure 1).
Laboratory testing was significant for a positive anti-nuclear antibody test at 1:160 (speckled pattern) and elevated anti-Ro/SSA antibody at 4.2 (reference range [RR]: <1.0 is negative); anti-La/SSB antibody, rheumatoid factor, thyroid-stimulating hormone, creatinine kinase and serum IgG4 level were all within normal limits. Her C-reactive protein (CRP) was normal, but her erythrocyte sedimentation rate (ESR) was elevated at 47 mm/hr (RR: 0–15 mm/hr).
A chest X-ray revealed no hilar lymphadenopathy.
Biopsy of the right submandibular gland displayed florid intralobular storiform fibrosis, and chronic lymphoplasmacytic infiltrate with an IgG4 to IgG ratio of 1:4 and focally increased IgG4 plasma cells: 14 per high-powered field (see Figure 2). Although obliterative phlebitis was not observed and the ratio of IgG4/IgG cells was not >40%, the overall histomorphological feature was suggestive of IgG4-related sialadenitis. The patient was started on prednisone, resulting in improvement of her submandibular swelling and arthralgias.
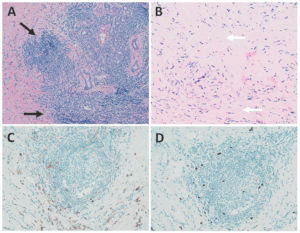
FIGURE 2: Right submandibular gland biopsy: (A) H&E staining with marked lymphoplasmacytic infiltrates (black arrows) without evidence of obliterative phlebitis; (B) fibrosis (white arrows) arranged in a swirling pattern known as storiform fibrosis, which is a hallmark feature of IgG4-RD; immunohistochemistry showed focally increased IgG+ (brown cytoplasmic staining); (C) IgG4+ (brown cytoplasmic staining); and (D) plasma cells with a ratio of ~4:1. The key histologic features of IgG4-RD are dense lymphoplasmacytic infiltrate, storiform-patterned fibrosis, obliterative phlebitis and infiltration of IgG4+ plasma cells with ratio of IgG4/IgG cells >40%, and >10 IgG4+ plasma cells/high-powered field in biopsy tissue sample. (Click to enlarge.)