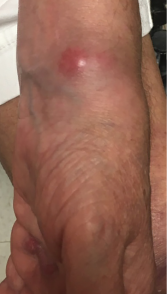
Figure 1. A lesion present on the patient’s left wrist at a clinic appointment in September 2018.
A 61-year-old white woman presented to our rheumatology clinic in New England to establish care in early June 2018, following a move from Texas. She reported a medical history of inflammatory bowel disease, uveitis and seronegative inflammatory arthritis, which was difficult to control and required the use of multiple medications.
At her initial visit, she reported taking 400 mg of hydroxychloroquine daily, 20 mg of methotrexate weekly, 5 mg of tofacitinib twice per day, 90 mg of ustekinumab weekly and 10 mg of prednisone daily. Ustekinumab was discontinued at that time due to a concern for excessive immunosuppression when used in combination with tofacitinib.
In early August 2018, she presented with new left wrist pain and swelling. Her exam was notable for diffuse metacarpophalangeal joint synovial thickening bilaterally and dorsal right wrist swelling with decreased flexion and extension due to pain. Her prednisone dose was briefly increased from 10 mg to 20 mg daily for the possibility that her wrist pain might represent an acute flare of inflammatory arthritis in the setting of discontinuation of ustekinumab.
In mid-August 2018, she underwent L5 decompressive lumbar laminectomy for central canal lumbar stenosis with radicular symptoms. Her course was complicated by postoperative fever. Urine showed greater than 100,000 cfu/mL Escherichia coli, for which she received a course of nitrofurantoin. By the end of August, she had fever recurrence. Computerized tomography (CT) scan of the lumbar spine showed a fluid collection concerning for abscess. Fluid aspirate was notable for pan sensitive Staphylococcus capitis, which grew only in broth media. She was started on six weeks of ceftriaxone and her remaining anti-rheumatic medications were discontinued; a prednisone taper was also initiated. Thereafter, she had no fever recurrence.
At her September 2018 rheumatology follow-up, she had profound swelling of her hands, especially the proximal interphalangeal joints, with wrist flexor and extensor tenosynovitis in both hands. Her left wrist showed a small, cystic-appearing lesion (see Figure 1). Her hydroxychloroquine was restarted and prednisone increased to 40 mg daily.
M. kansasii most typically causes pulmonary disease; however, it may also rarely manifest as skin & soft tissue, skeletal or disseminated disease.
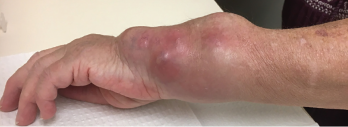
Figure 2. The patient’s left wrist at a clinic appointment in October 2018.
In October 2018, she returned to the rheumatology clinic acutely, due to rapidly progressive left wrist swelling and pain. The physical examination revealed a well-appearing patient with normal vital signs. The musculoskeletal exam was notable for marked swelling of the volar and ulnar aspects of the left wrist (see Figure 2).
Laboratory data revealed the values listed in Table 1 (see p. 60). Aspiration of the left wrist showed red, turbid fluid, 80,990 white blood cells/mcL (81% neutrophils) without crystals or organisms on Gram stain. Calcofluor white and acid-fast bacilli (AFB) stains were negative. A CT scan showed multiloculated fluid collections (see Figure 3) and associated bone destruction of the distal ulnar and radius (see Figures 4a and 4b), suspicious for osteomyelitis.
She underwent operative debridement of soft tissue and affected bone, with repeated specimen collection, with initial findings similar to the aspiration.
After six days, AFB culture grew an organism in liquid media; direct probe was negative for Mycobacterium tuberculosis and M. avium. In November 2018, the mycobacterial species was identified as M. kansasii, and she was started empirically on a daily, oral regimen of 1,000 mg of ethambutol, 300 mg of rifabutin and 250 mg of azithromycin.
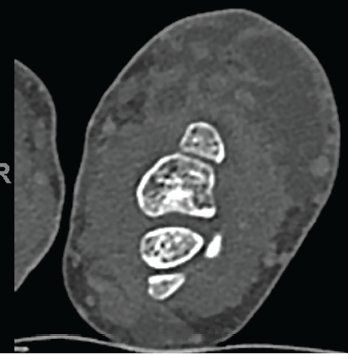
Figure 3. Multiloculated fluid collections in the dorsal subcutaneous tissue of the distal ulna seen on CT scan.
Given her escalating back pain and the finding of this organism, repeat CT imaging of the spine was performed to search for other infection sites. This showed degenerative changes of the thoracic spine and postsurgical changes of the lower lumbar spine, with interval improvement of the paraspinal soft tissues but no evidence of infection.
A drug sensitivity panel showed resistance to ciprofloxacin, but sensitivity to moxifloxacin, clarithromycin, amikacin, streptomycin, trimethoprim/sulfamethoxazole, linezolid, ethambutol, isoniazid, rifampin and rifabutin.
In December 2018, following slow but progressive postoperative wound healing, she developed increased purulent drainage (see Figure 5, p. 61), concerning for paradoxic worsening vs. treatment failure vs. bacterial superinfection. Antimycobacterials were temporarily held, and she underwent repeat debridement, specimens from which again showed M. kansasii, with broth-only culture showing coagulase-negative Staphylococcus.
Antimycobacterial therapy was subsequently reinitiated. She developed a whole body maculopapular rash attributed to rifabutin, so 5 mg/kg of intravenous amikacin three times per week was substituted, and the rash resolved. She completed a 15-week course of amikacin and was continued on azithromycin and ethambutol.
She developed nodular lesions on the left palm, two digits, as well as the right ventral wrist, in December 2018. White caseating material was expressible from these, and moxifloxacin was added to intensify the regimen in case these represented M. kansasii satellite lesions; she developed a prolonged QTc, so this was soon stopped.
She was continued on her two-drug regimen of azithromycin and ethambutol.
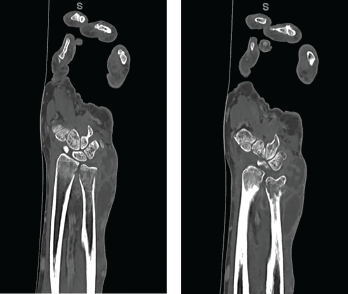
Figures 4a and 4b CT scan showing (a) a radial focus of bone destruction in the ulnar aspect of the distal left radius and (b) ulnar periostitis and bone destruction of the distal left ulna.
Plain films in late December 2018 showed progressive disease, so she underwent repeat operative debridement in early February 2019. Surgical cultures grew coagulase-negative Staphylococcus in three cultures (sensitive only to gentamycin, tetracycline and vancomycin), but did not grow M. kansasii. She was not started on additional antimicrobials because she had no symptoms of cellulitis or increased drainage from the wound. She had poor, postoperative wound healing, so she completed 45 sessions (five sessions per week) of hyperbaric oxygen therapy between March 2019 and May 2019.
The patient was seen urgently in late May 2019 for purulent drainage and wrist swelling, and underwent another operative debridement. Cultures showed Peptostreptococcus (tissue) and rare Corynebacterium (bone). She received vancomycin preoperatively and was discharged on doxycycline to complete a 14-day course. Subsequently, 200 mg of tedizolid daily was added to her regimen for both M. kansasii and Corynebacterium coverage.
Drainage from her wrist stopped in June 2019 (see Figure 6, opposite). At follow-up in November 2019, her left wrist wound was completely healed, without any further discharge. Her antimycobacterials were stopped, given a total of one year of treatment.
Discussion
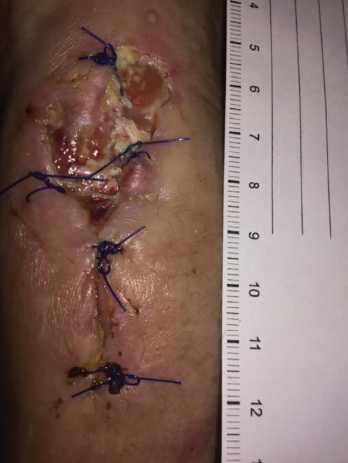
Figure 5. Increased purulent drainage from the left wrist in December 2018.
Mycobacterium kansasii is a slow-growing, photochromogenic, acid-fast nontuberculous mycobacterium (NTM) that was first described as a human pathogen in 1953.1,2 Even though the various NTM are common environmental organisms, M. kansasii has only been isolated from tap water in endemic areas, such as the southeastern and southern coastal states of the U.S.2,3 M. kansasii most typically causes pulmonary (i.e., upper lobe fibrocavity or chronic nodular) disease after organ transplantation, in patients with chronic lymphocytic leukemia and those infected by human immunodeficiency virus; however, it may also rarely manifest as skin and soft tissue, skeletal or disseminated disease.2
Disseminated cutaneous infections, characterized by multiple noncontiguous nodular draining skin lesions, are most commonly caused by rapidly growing NTM, such as M. chelonae and M. abscessus complex.2 These patients typically have underlying inflammatory diseases, such as rheumatoid arthritis, and are on chronic steroids, with doses as low as 5 mg of prednisone daily.4 Some slow-growing NTM, mostly commonly M. haemophilum and M. ulcerans, can cause a similar syndrome, but are typically in patients on higher dose steroids or multiple immunosuppressants.2
Biologics, especially anti-tumor necrosis factor (TNF) agents, have radically changed the treatment of inflammatory conditions. These agents have been associated with increased rates of reactivation of latent tuberculosis and de novo NTM infections, attributed to inhibition of the pro-inflammatory cytokine TNF involved in macrophage recruitment and formation of granulomas, which help contain and clear mycobacterial infections.4
Tofacitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes involved in signal transmission for cytokines. Tofacitinib primarily inhibits JAK1 and JAK3, and to some degree JAK2, which blocks the effects of interferon (IFN) γ, interleukin (IL) 6, IL-12 and IL-23; this inhibits the differentiation and action of T helper (Th) 1, Th2 and Th17 cells involved in adaptive and innate immune responses.5 Two cases have been reported of NTM pulmonary infections in rheumatoid arthritis patients receiving tofacitinib, in addition to other disease-modifying anti-rheumatic drugs (DMARDs), in a review of phase 2, 3 and long-term extension clinical trial data.6
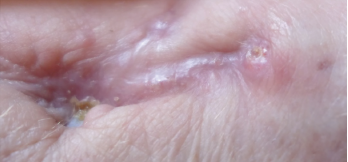
Figure 6. Close-up of the left wrist wound June 2019, with complete skin closure without further drainage.
Ustekinumab is a human IgG monoclonal antibody that binds the p40 protein subunit of IL-12 and IL-23. Both IL-12 and IL-23 are involved in the differentiation of CD4+ T cells to T helper (Th) lineage cells, Th1 cells (a source of IFN- γ) and Th17 cells (a source of IL-17 and TNF), which mediate cellular immunity.7 The risk of NTM infections, specifically M. kansasii, with the use of ustekinumab is unknown. A case was recently reported of an NTM infection (specifically M. abscessus) in a patient with Crohn’s disease treated with ustekinumab monotherapy, which had not been previously reported.8
Our patient was on dual biologic therapy for inflammatory bowel disease and seronegative inflammatory arthritis. Her reported duration of treatment with dual biologic therapy, methotrexate, hydroxychloroquine and prednisone before presentation was greater than 10 years. The use of dual biologic therapy is rare for inflammatory bowel disease and inflammatory arthritis, but has been used in cases where patients have refractory disease.9,10 These case series followed small numbers of patients for up to two years, with few reported adverse events.
A recent systematic review and meta-analysis of the use of two biologic DMARDs in rheumatoid arthritis did suggest an increased risk of serious infections and other adverse events.11 Currently, an open-label, phase 4 study is actively enrolling patients to assess triple therapy with vedolizumab, adalimumab and methotrexate in patients with newly diagnosed Crohn’s disease.10 It is unknown if similar studies looking at multiple biologic agents are underway for inflammatory arthritis patients.
Table 1: Initial Laboratory Data
| Variable | Result | Reference Range |
|---|---|---|
| White blood cell count | 7.9 X 103/μL | 4.0–9.5 X 103/μL |
| Hemoglobin | 11.3 gm/dL | 11.7–15.5 gm/dL |
| Hematocrit | 0.368 | 35.7–45.8% |
| Platelets | 303 X 103/μL | 145–357 X 103/μL |
| Sodium | 139 mmol/L | 135–145 mmol/L |
| Potassium | 3.5 mmol/L | 3.5–5.0 mmol/L |
| Chloride | 98 mmol/L | 98–107 mmol/L |
| Bicarbonate | 29 mmol/L | 22–31 mmol/L |
| Blood urea nitrogen | 18 mg/dL | 1–18 mg/dL |
| Creatinine | 0.67 mg/dL | 0.70–1.20 mg/dL |
| Glucose | 85 mg/dL | 65–199 mg/dL |
| C-reactive protein | 31.2 mg/L | ≤4.9 mg/L |
| Sedimentation rate | 32 mm/hr | 0–20 mm/hr |
Our patient had fallen on her hands before her move from Texas. Minor skin breakdown may have served as a point of inoculation that resulted in M. kansasii infection, given its prevalence in tap water in southern states.3 The concomitant use of methotrexate and prednisone with tofacitinib and ustekinumab likely increased her risk of more serious infection. Despite the number of immunosuppressive medications, our patient had very poor control of her inflammatory arthritis symptoms, yet was on a stable regimen for over 10 years without trials of other agents. Coordination between her specialists may have better targeted the organ systems affected by her inflammatory processes, resulting in a more favorable balance of infection risk and symptom control.
Conclusion
With the increasing number of NTM infections in patients on immunosuppressive medications, it is important to consider these pathogens in an infectious differential diagnosis. Molecular techniques have expedited the diagnosis and identification of NTM infections.2 It is, therefore, prudent to consider early, aggressive diagnostic evaluation of immunocompromised patients for chronic syndromes that could represent an atypical pathogen.
 Kayleigh A. Sullivan, MD, MA, MPH, completed her internal medicine residency at Dartmouth-Hitchcock Medical Center, N.H. She is an integrated geriatrics and palliative medicine fellow at the Icahn School of Medicine, Mount Sinai, N.Y.
Kayleigh A. Sullivan, MD, MA, MPH, completed her internal medicine residency at Dartmouth-Hitchcock Medical Center, N.H. She is an integrated geriatrics and palliative medicine fellow at the Icahn School of Medicine, Mount Sinai, N.Y.
 Nicole M. Orzechowski, DO, is an associate professor of medicine at the University of North Carolina at Chapel Hill and medical director of the Rheumatology Specialty Clinic.
Nicole M. Orzechowski, DO, is an associate professor of medicine at the University of North Carolina at Chapel Hill and medical director of the Rheumatology Specialty Clinic.
 Elizabeth A. Talbot, MD, is a professor of medicine at Geisel School of Medicine at Dartmouth College and the medical director of the NonTuberculous Mycobacterial Infections Clinic. She also serves as New Hampshire’s deputy state epidemiologist.
Elizabeth A. Talbot, MD, is a professor of medicine at Geisel School of Medicine at Dartmouth College and the medical director of the NonTuberculous Mycobacterial Infections Clinic. She also serves as New Hampshire’s deputy state epidemiologist.
Disclosures
Dr. Talbot is a consultant for Oxford Immunotec, the maker of T-Spot.TB, and a consultant with participation in an advisory board for Insmed, the maker of Arikayce.
References
- Buhler VB, Pollak A. Human infection with atypical acid-fast organisms: Report of two cases with pathologic findings. Am J Clin Pathol. 1953 Apr;23(4):363–374.
- Brown-Elliott BA, Wallace RJ. Infections caused by nontuberculous mycobacteria other than Mycobacterium avium complex. In Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease. 9th ed. Philadelphia, PA: Elsevier; 2020:3049–3058.
- Steadham JE. High-catalase strains of Mycobacterium kansasii isolated from water in Texas. J Clin Microbiol. 1980 May;11(5):496–498.
- Brown-Elliott BA, Philley JV. Rapidly growing mycobacteria. Microbiol Spectr. 2017 Jan;5(1).
- Roach DR, Bean AG, Demangel C, et al. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002 May 1;168(9):4620–4627.
- Laurence A, Gadina M, O’Shea JJ. Protein kinase antagonists in therapy of immunological and inflammatory diseases. In Rich RR, Fleisher TA, Shearer WT, et al., eds. Clinical Immunology: Principles and Practice. 5th ed. Philadelphia, PA: Elsevier; 2019:1185–1196.
- Winthrop KL, Park SH, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016 Jun;75(6):1133–1138.
- Chatham WW. Biological modifiers of inflammatory diseases. In Rich RR, Fleisher TA, Shearer WT, et al., eds. Clinical Immunology: Principles and Practice. 5th ed. Philadelphia, PA: Elsevier; 2019:1197–1210.
- Shim HH, Cai SCS, Chan W, et al. Mycobacterium abscessus infection during ustekinumab treatment in Crohn’s disease: A case report and review of the literature. J Crohns Colitis. 2018 Nov;12(12):1505–1507.
- Thibodeaux Q, Ly K, Reddy V, et al. Dual biologic therapy for recalcitrant psoriasis and psoriatic arthritis. JAAD Case Rep. 2019 Oct 10;5(10):928–930.
- Le Berre C, Loeuille D, Peyrin-Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol. 2019 Mar;17(4):794–796.
- Boleto G, Kanagaratnam L, Dramé M, et al. Safety of combination therapy with two bDMARDs in patients with rheumatoid arthritis: A systematic review and meta-analysis. Semin Arthritis Rheum. 2019 Aug;49(1);35–42.


