International, evidence-based consensus treatment guidelines for iMCD suggest tocilizumab with or without glucocorticoids as a first-line therapy for both iMCD-TAFRO & iMCD-NOS.
Discussion
Castleman disease is a heterogeneous LPD characterized by lymph node hyperplasia. Patients with Castleman disease present with solitary (unicentric) or multicentric lymphadenopathy. Around 50% of multicentric Castleman disease cases are driven by HHV-8 infection, while the etiology of the remaining half of HHV-8 negative/iMCD cases is unknown.
iMCD-TAFRO is a distinct clinical phenotype.2 A case report of iMCD-TAFRO was first described in Japan in 2010, but cases have been reported from all over the world.3 Patients with iMCD-TAFRO often have a poor prognosis; when compared with other forms of iMCD, iMCD-TAFRO is more more likely to be associated with hepatic and renal dysfunction, and a more aggressive clinical course.1
Diagnostic criteria for iMCD-TAFRO were proposed in 2019.4 A diagnosis requires all three items in the major criteria category and at least two of four items in the minor criteria category.
The major criteria include:
- Anasarca;
- Thrombocytopenia; and
- Evidence of systemic inflammation (i.e., fever of unknown etiology and elevated serum CRP).
Minor criteria include:
- Characteristic lymph node pathology;
- Reticulin myelofibrosis;
- Organomegaly; and
- Progressive kidney injury.
When diagnosing iMCD-TAFRO, the following should be excluded:
- Malignancies, including lymphoma;
- Autoimmune disorders, including SLE, Sjögren’s syndrome and ANCA-associated vasculitis;
- Infectious diseases;
- POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes);
- Liver cirrhosis; and
- Thrombotic microangiopathy.
When evaluating patients with any of these diagnoses, however, the presence of atypical features should prompt you to think of iMCD-TAFRO.
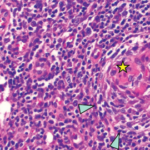
To establish a diagnosis, criteria proposed in 2015 required a lymph node biopsy, which should demonstrate the atrophy of germinal centers with enlarged endothelial cells nuclei, proliferating endothelial venules in the interfollicular zone and a few mature plasma cells.5 However, the requirement for a lymph node biopsy establish a diagnosis of iMCD-TAFRO was included as a minor, rather than a major, diagnostic criterion because the presence of anasarca and thrombocytopenia may make it difficult to perform a lymph node biopsy.
The pathogenesis of iMCD-TAFRO remains unclear.2 Some differences between iMCD-TAFRO and iMCD not otherwise specified (iMCD-NOS) have been reported. iMCD-TAFRO seems to be driven by IL-2, which causes a systemic capillary leak syndrome characterized by fluid retention, renal failure and thrombocytopenia.6 iMCD-TAFRO tends to be associated with more modest elevations of serum IL-6 and higher VEGF levels than those found in iMCD-NOS.7,8 Peritoneal levels of IL-6 and VEGF have reportedly been elevated in iMCD-TAFRO, which may contribute the development of this disease.9 Elevated serum platelet-derived growth factor level has also been reported in iMCD-TAFRO and may be associated with myelofibrosis.10
In addition, some similarities between iMCD-TAFRO and lupus have been reported. B cell and T cell activation appear to play a pathogenic role in iMCD-TAFRO which may explain the response of iMCD-TAFRO to both rituximab and cyclosporine A.2 Further, regarding epigenetic alternation, a DNA methyltransferase 3A mutation has been observed in patients with iMCD-TAFRO and SLE.11,12
Differentiating iMCD-TAFRO and lupus is critical to determine the appropriate therapeutic strategy. Approximately 10% of lupus cases meet the diagnostic criteria of iMCD-TAFRO.13 The acute and subacute manifestations of SLE and those of iMCD-TAFRO may not be distinguishable clinically, because both can be associated with systemic inflammation, effusions, cytopenias and generalized lymphadenopathy.
In a retrospective study, patients diagnosed with SLE who also met the diagnostic criteria for iMCD-TAFRO were more likely to be older, anti-dsDNA antibody negative and have higher titers of serum C-reactive protein than patients with SLE. Thrombocytopenia associated with iMCD-TAFRO was also less responsive to therapy and required longer treatment courses to achieve remission.
Therefore, physicians should consider a diagnosis of iMCD-TAFRO when evaluating a patient with SLE who is older or has a limited response to immunosuppressive therapy.
The ideal treatment for iMCD-TAFRO has not yet been established.
Clinical trials are challenging due to the rarity of patients with this disease. However, international, evidence-based consensus treatment guidelines for iMCD recommend tocilizumab with or without glucocorticoids as a first-line therapy for both iMCD-TAFRO and iMCD-NOS. In tocilizumab-resistant cases, cyclosporine A may be considered, particularly for refractory peritoneal effusion and thrombocytopenia.7
Based on retrospective research and expert opinion, the 2015 Japanese proposal of treatment strategy for iMCD-TAFRO describes glucocorticoids as first-line therapy, followed by cyclosporine A as second-line therapy—barring contraindications for cyclosporine A (e.g., renal dysfunction). If cyclosporine A is contraindicated, tocilizumab and rituximab may be considered.
Plasma exchange, cyclophosphamide (potentially as part of CHOP [cyclophosphamide, doxorubicin, vincristine and prednisolone]) and proteasome inhibitors, such as thalidomide and lenalidomide, are suggested for select patients.5
In a case series, three patients with tocilizumab-resistant iMCD-TAFRO achieved long-term remission with sirolimus, an inhibitor for mechanistic target of rapamycin (mTOR) that regulates VEGF expression and T cell activation.8
A case series reported tacrolimus may also be effective.14 Cyclosporine A and tacrolimus are both calcineurin inhibitors that regulate IL-2 transcription, leading to T cell inactivation.
In our case, however, cyclosporine A proved superior in efficacy over tacrolimus. Although further investigations are required, differences in drug delivery (i.e., penetrance into the bone marrow and effusions) and its effect on transforming growth factor β production, which increases platelet counts, may explain why cyclosporine A may be a superior therapy for iMCD-TAFRO.