Gout is the most common type of inflammatory arthritis in adults, and it typically occurs in men over the age of 50. When gout presents in younger patients or in women, this should warrant consideration of secondary causes. We describe an unusual genetic cause of tophaceous gout in a young, premenopausal woman.
Case Report
In 2020, a 31-year-old Black woman presented to the hospital with the chief complaint of numbness and tingling in her hands, feet and face, and was admitted for symptomatic hypocalcemia with a calcium level of 5 mg/dL (reference range [RR]: 8.6–10.2 mg/dL) and ionized calcium of 0.59 mmol/L (RR: 1.18–1.33 mmol/L). A rheumatologist was consulted for a complaint of right knee and left ankle pain and swelling for four days.
The patient complained of a sharp, constant discomfort, 10/10 in intensity in the right knee, with difficulty walking and finding a position of comfort. Despite receiving 0.2 mg of hydromorphone, 2 mg of morphine and 1 g of acetaminophen every six hours, she had no analgesic relief. She denied any trauma or recent injury, fevers, chills, upper respiratory symptoms, abdominal discomfort or other arthralgias; however, she did note some loose stools.
Of note, the patient had been hospitalized two weeks before with a similar presentation of symptomatic hypocalcemia following a parathyroidectomy. At that time, she also had inflammatory arthritis of both ankles. No arthrocentesis was performed because she had been diagnosed with crystal-proven gout in 2016. On the previous admission, a 10-day course of 40 mg of prednisone was initiated. She had been on febuxostat since 2016, but she took this drug intermittently due to issues with diarrhea.
The patient’s pertinent past medical history included her initial presentation to our rheumatology clinic in 2012, at age 23, with bilateral foot and ankle pain. History at that time was pertinent for Bartter syndrome, diagnosed during infancy given failure to thrive and metabolic abnormalities, including metabolic alkalosis, hypomagnesemia, hypokalemia and elevated renin, with normal aldosterone levels. She had a presumed diagnosis of gout since the age of 21 based on recurrent ankle and toe pain, with inflammation and hyperuricemia; however, no joint aspirations had been performed. For these episodes, she was treated with naproxen and prednisone, with no chronic therapy.
At least one other case reports the association between gout & Gitelman syndrome.
On her initial visit in 2012, a physical exam revealed tenderness to palpation, warmth and erythema in both first metatarsophalangeal (MTP) joints. She also had tenderness to palpation over the left olecranon process with mild warmth, but no swelling. On this occasion, aspiration of the first right MTP joint and left olecranon bursa was attempted, and no crystals were identified. A laboratory evaluation demonstrated no hematologic abnormalities; however, a metabolic profile revealed a creatinine of 1.5 mg/dL (RR: 0.5–1.10 mg/dL) with a glomerular filtration rate (GFR) of 52 and, given her pediatric diagnosis of Bartter syndrome, a consultation with a nephrologist was obtained.
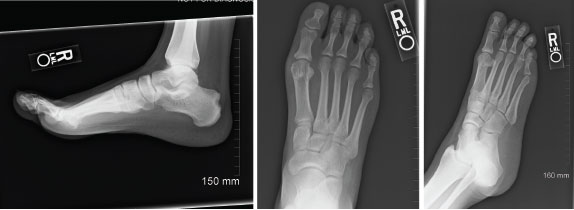
Figure 1: Radiographs of Right Foot, 2012
Imaging demonstrates a large erosion involving the posterior margin of the right calcaneus, near the Achilles insertion. Erosive changes involving the first metatarsophalangeal joints are noted, consistent with an inflammatory arthritis.
The nephrologist believed a diagnosis of Gitelman syndrome was appropriate based on her metabolic findings at that time.
She tested negative for rheumatoid factor and anti-citrullinated peptide antibodies, and a uric acid level of 10.9 mg/dL was documented. Imaging of her feet revealed erosions in both first MTP joints and a large right calcaneal erosion (see Figure 1). Conventional radiographic imaging revealed right-sided sacroiliitis, although she had not complained of back pain.
At that time, she was felt to have an inflammatory, erosive arthritis, and in combination with the radiographic findings of sacroiliitis, a diagnosis of spondyloarthropathy was made. She was started on a tumor necrosis factor-α inhibitor.
Four years later, in 2016, the patient was hospitalized with polyarthritis. A right knee arthrocentesis was performed, revealing intra- and extracellular monosodium urate crystals. Around this same time, tophi of the elbows and third distal interphalangeal joint were reported.
For the first time, this 27-year-old premenopausal female had a crystal-proven diagnosis of tophaceous gouty arthritis. She was treated with corticosteroids and discharged from the hospital. On a subsequent outpatient visit, 100 mg of daily allopurinol was initiated, with a concomitant prednisone taper.
One month later the patient was hospitalized in the intensive care unit with DRESS (i.e., drug reaction with eosinophilia and systemic symptoms), based on fever, rash and time frame of recently starting allopurinol, which was subsequently discontinued.
Shortly thereafter, her uric acid level was elevated at 13.2 mg/dL with a creatinine of 1.5 mg/dL and estimated GFR of 50. Febuxostat (40 mg per day) and colchicine (0.6 mg daily) were initiated. On subsequent follow-up visits, her uric acid levels did decline, but never below goal levels of <6g/dL, and over the next four years she continued to experience recurrent gout attacks.
At various times during 2016–2020, laboratory testing demonstrated hypercalcemia, with levels between 10.4 and 11.2 mg/dL. This was initially presumed to be due to secondary hyperparathyroidism from her chronic renal disease. In 2016, an endocrinologist made a diagnosis of primary hyperparathyroidism based on significantly elevated parathyroid hormone levels of 2,471 pg/mL at maximum (intact parathyroid hormone RR: 15–72 pg/mL). A parathyroid ultrasound revealed bilateral parathyroid adenomas. She underwent a parathyroidectomy in 2020.
Case Summary
The patient’s complete medical history included:
- Gitelman syndrome with chronic, stage IV kidney disease;
- Crystal-proven tophaceous gout, with recurrent attacks of gouty arthritis over many years;
- Possible spondyloarthropathy;
- Anemia due to chronic kidney disease;
- Obesity; and
- Primary hyperparathyroidism.
Her surgical history included a salpingostomy for an ectopic pregnancy in 2016 and the subtotal parathyroidectomy in 2020.
She is married with two children, and she denied any tobacco or alcohol use. Her family history was significant for fibromyalgia in her mother, but no family history of gout.
Her outpatient medications included 5 mg of amiloride daily, 500 mg of calcium carbonate, 2,850 mg of calcium citrate three times a day, 40 mg of febuxostat daily, 0.6 mg of colchicine three times per week and 50 mg/0.5 mL of golimumab via subcutaneous monthly injections. Current inpatient medications consisted of calcium gluconate and magnesium replacement.
Her vital signs from the current inpatient admission demonstrated a temperature of 99.4ºF and a blood pressure of 151/74 mmHg. A physical exam revealed an obese, uncomfortable-appearing woman with a warm right knee with a moderate effusion and very limited range of motion due to pain. Her left ankle was also warm and painful, with a reduced range of motion.
Her white blood cell count was 13.8K/uL, and her creatinine level was 2.75 mg/dL (eGFR 24). Two weeks previously, her uric acid level was 8.5 mg/dL, and that test was not repeated.
An arthrocentesis of the right knee was performed, yielding a total cell count of 35,800K/uL with 97% neutrophils and identification of negatively birefringent needle-shaped crystals consistent with gouty arthritis.
Treatment included a prolonged course of steroids and continuation of febuxostat. In the three months following her hospitalization and parathyroidectomy, she was hospitalized six more times with symptomatic hypocalcemia and recurrent gout attacks.
Discussion
Gitelman syndrome is a rare, autosomal recessive genetic defect of the sodium chloride transporter, resulting in a salt-wasting nephropathy with complications of hypomagnesemia, hypokalemia and hypocalciuria. The defective transport mimics that of a thiazide diuretic, targeting the distal tubule. This results in enhanced salt and hydrogen ion secretion, leading to metabolic alkalosis with stimulation of the renin-angiotensin-aldosterone system, and reabsorption of uric acid through the urate transporter URAT1.1-3
Additionally, defects in the uric acid transporter SLC17A3 have been described in Gitelman syndrome, and this may also contribute to hyperuricemia.2,4-6
Treatment for Gitelman syndrome consists of correcting the electrolyte derangements by blocking the sodium-potassium exchange through the use of non-steroidal anti-inflammatory drugs and potassium-sparing diuretics, such as spironolactone or amiloride.4
Etiologies of Hyperuricemia
| Underexcretion of uric acid | Overproduction of uric acid |
|---|---|
| Inherited: Gitelman syndrome, autosomal dominant tubulointerstitial kidney disease | Inherited monogenic defects in purine, sugar or adenosine triphosphate metabolism or other genetic abnormalities: Hypoxanthine-guanine phosophoribosyltransferase deficiency, PRP overactivity, glucose-6-phosphate dehydrogenase deficiency, Down syndrome |
| Renal: Insufficiency, lead nephropathy | Increased cell turnover: Hemolysis, lymphoproliferative disorder, myeloproliferative disorders, psoriasis |
| Cardiac: Congestive heart failure | Increased purine ingestion: Meat, seafood |
| Acidotic states: Ketoacidosis, lactic acidosis | Iatrogenic: Cytotoxic medication (contributing to tumor lysis syndrome) |
| Endocrinologic: Hyperparathyroidism, hypothyroidism | |
| Autoimmune: Sarcoidosis | |
| Iatrogenic: Cyclosporine, thiazide diuretics, loop diuretics, pyrazinamide, ethanol, laxatives |
Gitelman syndrome causes chronic hypomagnesemia, which has been associated with calcium pyrophosphate deposition disease, because magnesium increases calcium pyrophosphate (CPP) crystal solubility and acts as a cofactor for many enzymes that degrade pyrophosphate, which is the anionic component of the CPP crystal.5-7
Our patient had no radiographic evidence of chondrocalcinosis, nor did she have positively birefringent crystals in any of her synovial fluid samples. We have found no literature that supports an association between hypocalcemia and hyperuricemia. However, a recent study demonstrated an inverse relationship between magnesium and uric acid levels, so that chronic hypomagnesemia may contribute to hyperuricemia.8
Although metabolic abnormalities are recognized as associations with calcium pyrophosphate deposition disease, this case illustrates that electrolyte derangements may contribute to the development of other crystalline arthropathies.7
Gitelman syndrome is rare; nonetheless, it should be recognized as another metabolic cause of hyperuricemia and gout based on renal underexcretion of uric acid. In addition, it should be considered as a diagnosis in younger, premenopausal females, who usually are protected from gout by the enhancement of renal acid excretion with estrogen.1,2,9
Our patient had several comorbidities (e.g., obesity and chronic renal insufficiency) that could have contributed to hyperuricemia; however, her hyperuricemia was out of proportion to the degree of azotemia.1,2,9 She also had hypercalcemia secondary to primary hyperparathyroidism, which can result in an increased uric acid reabsorption and hyperuricemia.
Studies have demonstrated that uric acid levels decrease following a parathyroidectomy or, contrastingly, that levels increase with teriparatide, a parathyroid hormone agonist.1,10,11
Gitelman syndrome and concomitant hyperparathyroidism are rare, with only a few cases cited in literature.12 Although Gitelman syndrome mutations mimic thiazide diuretics, it is rare to see hypercalcemia due to hypomagnesemia impacting calciotropic hormones.12
In retrospect, we question our patient’s 2012 diagnosis of spondyloarthritis. Gout is well documented to affect peripheral joints; however, although rare, it can also have axial involvement. A small number of case reports describe gout at the sacroiliac joint.13 This can be difficult to diagnose on plain radiographs.7,14 Radiographs of the pelvis in 2012 initially revealed suspected right-sided sacroiliitis. Later, in 2020, computed tomography (CT) of the abdomen and pelvis revealed bilateral sacroiliac joint erosions and resorption, and a diffuse sclerotic appearance of the osseous structures. We believe this is more consistent with her hyperparathyroidism, metabolic derangements or possible tophi.7
No dual-energy CT scan was performed. Her initial imaging of MTP and calcaneal erosions were likely most representative of gouty erosions, rather than enthesitis due to spondyloarthritis (see Figure 2). Tumor necrosis factor-α inhibitors have not been associated with hyperuricemia.
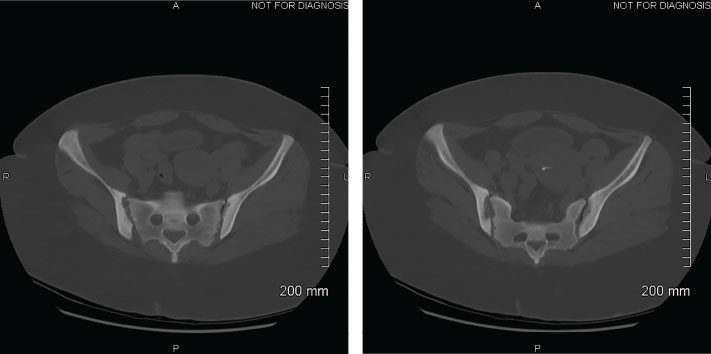
Figure 2: CTs of the Abdomen and Pelvis, Focus on the Sacroiliac Joints, 2020
Bilateral erosions of the sacroiliac joints with subchondral resorption/erosions that may be due to possible hyperparathyroidism or non-specific sacroiliitis are evident. The diffuse sclerotic appearance of imaged osseous structures is suggestive of renal osteodystrophy or sequela of prior metabolic derangement.
At least one other case report describes an association between gout and Gitelman syndrome.2 Historically, inherited defects in the salvage pathway (Lesch-Nyhan syndrome), phosphoribosyl pyrophosphate synthetase (PRP) overactivity, glucose-6-phosphate dehydrogenase deficiency or Down syndrome are thought to be the primary genetic causes for hyperuricemia due to urate overproduction from excess purine.1,2,9
In our patient, Gitelman syndrome was associated with premature, severe tophaceous gout through a pathway that is likely related to urate underexcretion and not overproduction due to a rare, genetic defect.1-6 This is particularly important because of the strong association of Gitelman syndrome with calcium pyrophosphate deposition disease, a common gout mimic.7
Conclusion
This case highlights the relationship between Gitelman syndrome and hyperuricemia, and it contributes to the limited number of case reports on Gitelman syndrome with gout and hyperparathyroidism.2,12 This case should reinforce and expand the paradigm of unusual genetic and metabolic causes of hyperuricemia, especially in younger patients who don’t typically meet the epidemiologic consideration for gout, particularly in young, premenopausal women.
Although rare, gout should be considered in patients with Gitelman syndrome.2
 Rebecca Lindsey Weiner, DO, is a first-year rheumatology fellow at the Medical College of Wisconsin in Milwaukee.
Rebecca Lindsey Weiner, DO, is a first-year rheumatology fellow at the Medical College of Wisconsin in Milwaukee.
 Ann K. Rosenthal, MD, FACP, is the Will and Cava Ross Professor of Medicine, Division of Rheumatology, Medical College of Wisconsin, and is chief of the Rheumatology Division and associate chief of staff for research and development at the Zablocki VA Medical Center.
Ann K. Rosenthal, MD, FACP, is the Will and Cava Ross Professor of Medicine, Division of Rheumatology, Medical College of Wisconsin, and is chief of the Rheumatology Division and associate chief of staff for research and development at the Zablocki VA Medical Center.
References
- Mount DB. Urate Balance. In UpToDate, Post TW (Ed), Waltham, Mass. (Accessed Oct. 19, 2020).
- Troster SM, Raizman JE, Rubin L. An unusual case of gout in a young woman with Gitelman Syndrome. J Rheumatol. 2016 Nov;43(11):2085–2087.
- El-Sheikh AAK, van den Heuvel JJM, Koenderink JB, et al. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008 Dec;155(7):1066–1075.
- Emmett M, Ellison DH. Bartter and Gitelman syndromes. In UpToDate, Post TW (Ed), Waltham, Mass. (Accessed Oct. 19, 2020).
- Naesens M, Steels P, Verberckmoes R, et al. Bartter’s and Gitelman’s syndromes: From gene to clinic. Nephron Physiol. 2004;96(3):p65–78.
- Simon DB, Nelson-Williams C, Bia, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996 Jan;12(1):24–30.
- Punzi L, Calò L, Schiavon F, et al. Chondrocalcinosis is a feature of Gitelman’s variant of Bartter’s syndrome. A new look at the hypomagnesemia associated with calcium pyrophosphate dihydrate crystal deposition disease. Rev Rhum Engl Ed. 1998 Oct;65(10):571–574.
- Cao J, Zhang J, Zhang Y, et al. Plasma magnesium and the risk of new-onset hyperuricaemia in hypertensive patients. Br J Nutr. 2020 Mar 26;1–8.
- Robinson PC. Gout—An update of aetiology, genetics, co-morbidities and management. Maturitas. 2018 Dec;118:67–73.
- Ponvilawan B, Charoenngam N, Ungprasert P. Primary hyperparathyroidism is associated with a higher level of serum uric acid: A systematic review and meta-analysis. Int J Rheum Dis. 2020 Feb;23(2):174–180.
- Hui JY, Choi JWJ, Mount DB, et al. The independent association between parathyroid hormone levels and hyperuricemia: A national population study. Arthritis Res Ther. 2012 Mar 10;14(2):R56.
- Wen YK. An unusual case of Gitelman’s syndrome with hypercalcemia. Ren Fail. 2012;34(2):241–243.
- Chen W, Wang Y, Li Y, et al. Gout mimicking spondyloarthritis: Case report and literature review. J Pain Res. 2017 Jun 29;10:1511–1514.
- Alqatari S, Visevic R, Marshall N, et al. An unexpected cause of sacroiliitis in a patient with gout and chronic psoriasis with inflammatory arthritis: A case report. BMC Musculoskelet Disord. 2018 Apr 20;19(1):126.

