Based upon these considerations, it has been suggested that varying the timing of glucocorticoid administration to coincide better with circadian rhythms could help improve therapy for RA.20 The scientific basis for this hypothesis is provided by the following three key points:
- Pain, fatigue, morning stiffness, and immobility are common symptoms affecting patient quality of life and the ability to stay gainfully employed.21
- The overnight rise in IL-6 and other proinflammatory cytokines is thought to initiate a cascade of events resulting in these symptoms.
- Preventing the nocturnal rise of IL-6 and other proinflammatory cytokines should be more effective than treating established symptoms. From this point of view, the conventional administration of glucocorticoids between 6 a.m. and 8 a.m. may not be optimal, because it is too late to target the effects of nocturnal proinflammatory stimuli (see Figure 1A).
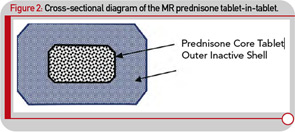
Although administering a standard glucocorticoid drug prior to the rise of cytokine synthesis and inflammatory activity could theoretically enhance efficacy, in practical terms, this approach would necessitate having the patient wake up during the night to take the drug, because conventional glucocorticoids have only a short half-life. Evidence that timing of exogenous glucocorticoid administration can improve treatment benefits was provided by Arvidson and co-workers in a study in the late 1990s. This study showed that low doses of prednisolone taken at 2 a.m. had more effect on morning symptoms of RA than achieved by the equivalent dose taken at 7:30 a.m. (see Figure 1B).17 However, having patients awake each night at about 2 a.m. is clearly not a feasible long-term treatment option. Therefore, a MR prednisone tablet was developed to enable prednisone chronotherapy for RA, in which the delivery of treatment is coordinated with biological rhythms (see Figure 1B). This new tablet releases prednisone approximately four hours after ingestion, (i.e., at approximately 2 a.m. if taken at bedtime) as indicated in Table 1. The MR prednisone is in a tablet-in-tablet dosage form, consisting of an immediate-release prednisone core tablet surrounded by an inactive outer tablet shell (see Figure 2). Prednisone release is triggered by penetration of gastrointestinal fluid into the tablet shell and is independent of the gastrointestinal milieu (like pH). The tablet strengths of 1, 2, and 5 mg are distinguished from one another by both color and debossing.