Aside from higher doses, the presence of renal failure, liver failure and concurrent use of CYP3A4 inhibitors could increase the risk of gastrointestinal side effects.28 Given how common statin use is in colchicine users, it is worthwhile to note the potential drug-drug interaction with certain statins that are substrates for CYP3A4 (e.g., atorvastatin and simvastatin) and consider replacing them with a statin that is not metabolized by CYP3A4 (e.g., pravastatin or rosuvastatin).28
Other side effects of importance to note include cytopenia, bone marrow suppression and vacuolar myopathy. These are quite rare.5,29
Unapproved Drugs Initiative & Cost
Despite being used successfully to treat gouty arthritis for a long time and its availability as a generic prescription drug in the U.S. since the 19th century, colchicine was not approved by the FDA until 2009. In 1938, the Food, Drug, and Cosmetic Act required all new drugs be approved by the FDA before being released to the market. In the 1960s, the agency embarked on evaluating the safety and efficacy of older drugs. While the FDA approved a combination pill containing colchicine and probenecid for use in gout, monotherapy of colchicine was not approved.
In 2006, the FDA launched the Unapproved Drugs Initiative, with a primary intention of documenting supporting data for several drugs that remained in the market continually, even before the agency began reviewing safety and effectiveness data in 1938.30 In exchange, the FDA offered market exclusivity to the first manufacturers of the product.
A clinical trial assessing the efficacy of colchicine in gout was completed, and the FDA approved colchicine under the brand name Colcrys for the treatment of acute
gout, granting Takeda Pharmaceuticals a three-year period of market exclusivity for gout and a seven-year exclusivity for use in FMF under the Orphan Drug Act.31 In 2010, the FDA ordered other manufacturers to stop manufacturing their products. In 2011, branded colchicine was the only formulation available in the U.S.32
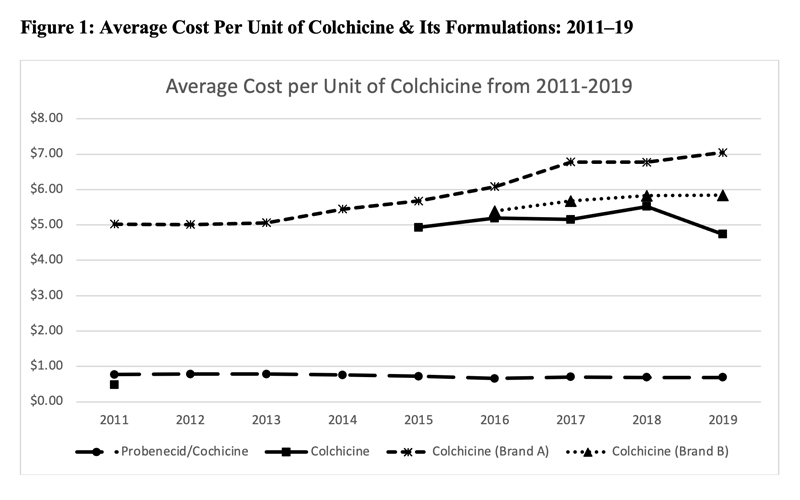
This led to a significant increase in the price of colchicine. Figure 1 (below) shows the average cost per unit of various formulations of colchicine as noted in the Centers for Medicare & Medicaid Services Medicare Part D spending files. Although data prior to 2011 were unavailable, the cost of generic colchicine in 2011 was $0.48. During the period of market exclusivity, the unit cost of branded colchicine soared to well over $5.
In 2015, generic colchicine was once again available in the market; however, despite the availability of alternative formulations of colchicine, the price of colchicine remained high.33,34