Pathogenesis of Behçet’s Syndrome
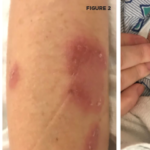
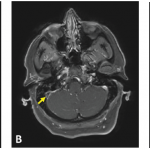
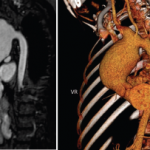
Although vascular injury is quite common in BS and can involve venous and arterial vessels of all sizes, frank necrotizing vasculitis of the small and medium-sized arteries as seen in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides is infrequent in patients with BS. Similarly infrequent are giant cells and immune complex–type cutaneous venulitis. These “negative” findings make the vascular involvement in BS unique. Furthermore, there is little evidence for vasculitis in some common lesions of BS. For example, there is no evidence for vascular injury in the papulopustular lesions of the skin and scanty evidence for a frank vasculitis in the CNS lesions. Conversely, the diffuse inflammatory disease in all layers of the large veins characteristically involving substantial segments of the vessel wall, the pulmonary artery aneurysms specific to this condition, and pseudoaneurysms of the large arteries (most probably secondary to vasculitis of the vasa vasorum) are unique findings.
The genetics of BS are, at present, uncertain. No clear Mendelian pattern has emerged. In endemic areas, approximately one in 10 families have more than one family member who is affected. The most consistent genetic association has been with HLA-B51, which explains only approximately 20% of the disease heritability.