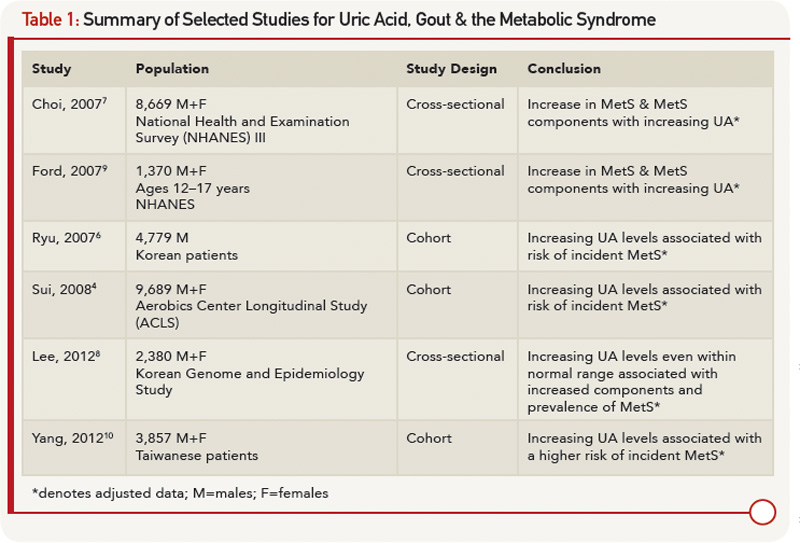
On the one hand, a physiologic case can be made for MetS leading to an increase in UA levels. On the other hand, mechanisms for increased UA levels causing MetS have also been described. Elevated levels of insulin increase UA reabsorption at the renal proximal tubule via stimulation of urate-anion exchanger URAT1 and the Na+-dependent anion co-transporter, leading to elevation in serum UA levels.11 Hyperinsulinemia may also diminish oxidative phosphorylation, resulting in elevated adenosine levels, which decrease renal UA excretion and increase UA production.7 Additionally, leptin levels increase with obesity; rising leptin is felt to be independently associated with and contribute to increasing UA levels via reduced renal excretion.12 Alternatively, UA levels could contribute to components of the MetS. Hyperuricemia may cause endothelial dysfunction and a reduction in endothelial nitric oxide (NO).13 NO is integral in promoting glucose influx to skeletal muscle; disruption to the action of insulin could lead to insulin resistance.13 Lastly, UA has been implicated in the proinflammatory milieu of adipose tissue. UA has been found in vitro to increase monocyte chemotactic protein-1 (MCP‑1), a proinflammatory adipokine, as well as decrease adiponectin, a known antiinflammatory and insulin sensitizer.14 Supporting the role of UA contributing to MetS, treatment with allopurinol in hyperuricemic obese mice decreases MCP-1, increases adiponectin, and improves insulin resistance.14
Fructose, found in sugar-sweetened soft drinks, has recently come under fire for its role in promoting hyperuricemia, gout and the MetS.15 Fructose metabolism increases UA through purine nucleotide degradation and de novo purine synthesis, setting the stage for UA-induced NO inhibition.15,16 Indeed, rats on a high-fructose diet treated with allopurinol or benzbromarone benefit from prevention or reversal of MetS components, providing interventional support to the idea that UA may be a cause of MetS.16 Figure 1 (right) demonstrates the bidirectional relationship between UA and the MetS as mediated through insulin resistance, obesity and renal excretion.
Diabetes
As described above, UA is associated with insulin resistance. Data also indicate a link between UA, gout and DM. A retrospective analysis demonstrates the risk of incident DM to be significantly higher in patients with hyperuricemia (UA >7), suggesting that 8.7% cases of new-onset DM are attributable to hyperuricemia.17 Prior prospective data report an even higher estimate of one-quarter of all DM diagnoses attributed to UA.18 Large prospective cohort studies, including the Framingham Heart Study and the Coronary Artery Risk Development in Young Adults, have confirmed UA to be a predictor for DM development.19,20 A cohort study notes a 78% increase in risk of impaired fasting glucose or DM when the highest UA quintile was compared with the lowest.21 Interestingly, although patients with gout appear to have an increased risk of DM, patients with DM tend to be protected from gout. In a cohort of men with high cardiovascular risk, having a diagnosis of gout is associated with a 1.3 times higher risk of incident DM.22 Conversely, a recent case-control study reports decreased incident gout with increasing hemoglobin A1c (HbA1c) values over 7% and DM duration, suggesting a protective role for DM.23
