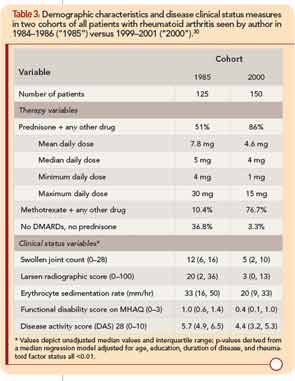
Substantially better clinical status was observed in all 150 RA patients seen by the author in 2000 compared to all 125 patients seen in 1985, according to MDHAQ function, swollen joint count, radiographic damage, erythrocyte sedimentation rate, and other measures (see Table 3, p. 22).30 In 1985, 51% of patients were taking prednisone with a mean daily dose of 7.8 mg/day, 10% were taking methotrexate, and 37% no DMARDs or prednisone. In 2000, 86% were taking prednisone at a mean dose of 4.6 mg/day, 77% were taking methotrexate, and only 3% were taking no DMARDs or prednisone (see Table 3).
The primary adverse events in patients treated with <5 mg/day prednisone were bruising and skin thinning. There were six new cases of hypertension over a mean of 5.2 years in 88 patients over 557 patient-years, five new cases of diabetes over a mean of 5.0 years in 105 patients over 632 patient-years, and four new cases of cataracts over a mean of 2.5 years in 103 patients over 617 patient-years (see Table 4). Mean (median) weight gain was 1.45 kg (1.13 kg) over 48 weeks. Weight gain was lowest in patients who began prednisone ≤3 mg/day (mean 0.44 kg), and greatest in patients who began prednisone >5 mg/day (mean 2.63 kg).25

All patients who were treated with prednisone at any dose were offered an opportunity to discontinue their prednisone, in a tapering schedule of 1 mg/month or 1 mg every two months, with a schedule to take, say, 3 mg on odd days and 2 mg on even days. Most patients tried this at least once, often several times, as many of their other physicians, and often their spouses, neighbors, and relatives all urged them to “stop taking that prednisone that Dr. Pincus is giving you.” The main reason expressed by patients for nonparticipation in the prednisone withdrawal clinical trial was that they had already attempted to discontinue prednisone many times and wished not to be randomized to placebo.13 Some elderly women reported having bruises and skin thinning, and related that they were forced to wear long sleeves so that people “would not think that my husband was beating me.” Nonetheless, most patients elected to continue long-term prednisone in doses of 1–4 mg/day, as a “trade-off” of minimal adverse events appeared justified to maintain functional status and well-being.