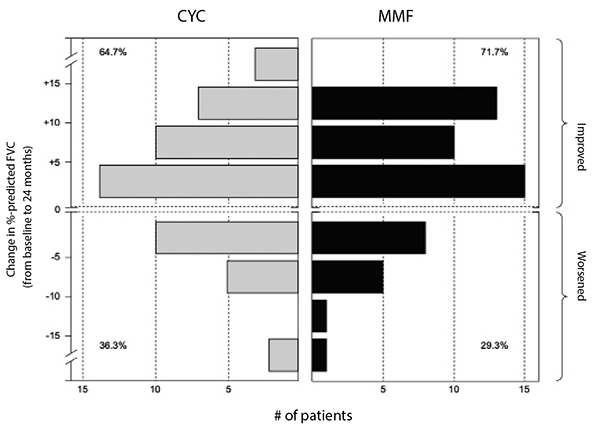
(click for larger image) Figure 2: Frequency distribution of changes from baseline to 24 months in FVC percent-predicted by treatment arm in SLS II.
Where these two drugs—mycophenolate mofetil and oral cyclophosphamide—diverged, however, was in their safety and tolerability profiles.6
Leukopenia (30 vs. four patients) and thrombocytopenia (four vs. zero patients) occurred significantly more frequently with cyclophosphamide than with mycophenolate. Frequent dose interruptions and titration setbacks occurred in the cyclophosphamide arm, but problems titrating mycophenolate were rare. Fewer patients on mycophenolate mofetil than on cyclophosphamide prematurely withdrew from the study drug (20 vs. 32) or met prespecified criteria for treatment failure (zero vs. two, defined as an absolute decline in FVC percent-predicted from baseline of 15%).
Taken together, the results of SLS II demonstrated mycophenolate mofetil is safer and better tolerated than cyclophosphamide.6 Although there was no difference in efficacy between these agents, it is plausible that advantages from mycophenolate were not detected due to initiation of alternative therapy in the second year of the study in the cyclophosphamide arm. For example, 12 of the 16 patients in the cyclophosphamide arm who prematurely stopped treatment but returned for the final study visit reported they had started an alternative therapy for their SSc-ILD (the most common agent used was mycophenolate).
Although there are no placebo-controlled trials for mycophenolate, an analysis comparing the mycophenolate arm of SLS II with the placebo arm of SLS I demonstrated that treatment with mycophenolate (compared with placebo) was associated with a significant improvement in the course of FVC percent-predicted, diffusing capacity, skin score and dyspnea over 24 months, even after adjusting for baseline disease severity.7 Moreover, patients assigned to mycophenolate suffered substantially fewer study drug withdrawals and serious adverse effects compared with patients assigned to placebo.7
Study #3
Based on the aforementioned studies, mycophenolate has emerged as a first-line treatment option for patients with SSc-ILD. However, as already noted, not all patients with SSc-ILD experience an improvement in their FVC percent-predicted when treated with mycophenolate, or with cyclophosphamide. In addition, the results of long-term follow-up analysis of patients who participated in SLS I and II revealed the leading cause of death in these patients was respiratory failure due to underlying ILD.8 Twelve years after the first patient was randomized in SLS I, 42% of participants had died (cyclophosphamide: 38; placebo: 28).8 Eight years after the first patient was randomized in SLS II, 21% of participants had died (cyclophosphamide: 16; mycophenolate: 14).8 Clearly, treatment with immunosuppression, at least for one to two years, is insufficient to halt the devastating clinical impact of ILD in certain patients with SSc.
Both cyclophosphamide and mycophenolate target predominantly inflammatory pathways implicated in SSc-ILD pathogenesis. Agents that specifically target fibrotic pathways may offer additional therapeutic benefit in this patient population, especially if combined with the well-tolerated immunosuppressant agent, mycophenolate. SLS III was specifically designed to test the hypothesis that the addition of anti-fibrotic therapy (i.e., pirfenidone) to a cytotoxic, immunosuppressant agent (i.e., mycophenolate) might lead to an improved treatment response compared with using an immunosuppressant agent alone.
