I have always been dissatisfied with the apparent dichotomy between researchers and clinicians; this is a false divide. Yes, research includes laboratory-based investigation, clinical trials, and retrospective and prospective studies of disease entities, among other pursuits. However, clinicians also contribute to research by sharing clinical data and through observations of their patients. Clinicians’ daily contributions ensure we have the opportunity to learn from every patient seen in rheumatology practice, leading to the development of ideas for improvements in clinical care, subsequent studies into disease mechanism and clinical trials to test new interventions.
As rheumatologists and rheumatology professionals, we all contribute to this cycle of knowledge generation that advances rheumatology practice every day.
To move our specialty forward, rheumatologists and rheumatology professionals need the right tools to conceive needed studies and participate in research. How we accomplish this, without adding more work to a clinician’s already full plate, is challenging. I am proud to say the ACR’s RISE registry provides one such tool.
Jinoos Yazdany, MD, MPH, chair of the ACR’s Committee on Registries and Health Information Technology (RHIT), and her committee colleagues have ensured RISE captures unique data that are allowing rheumatology professionals to rethink research, as well as patient care, for the better. RISE exists to capture as much critical data as possible, helping clinicians and more traditional researchers put those data into a meaningful context.
Data with Context
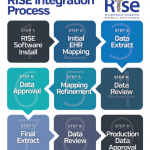
The RISE registry is a Qualified Clinical Data Registry (QCDR) run by the ACR and led by the RHIT committee. RISE was initially conceived as a means to create a digital data network that allowed broad data sharing across rheumatology practices. A key early goal was also to make it easier for clinicians to participate in quality measurement programs required by the Centers for Medicare & Medicaid Services (CMS).
The RISE team works with practices across the country to connect their electronic health record (EHR) systems to the registry. When RISE users enter data into a patient’s record within their EHR, these data are automatically extracted to the registry. The data then populates the user’s RISE dashboard, showing their performance on metrics that indicate standard measures of quality.
RISE users can also see how their performance on these metrics compares with that of peers, as well as with national benchmarks published by the CMS. In doing so, RISE users can identify performance gaps and target their efforts strategically toward improvements in patient care.